Louis Columbus, Principal, IQMS03.15.18
Medical device manufacturers are relying on four top priorities to lead their companies today: improving product quality, streamlining regulatory compliance reporting, optimizing product costs, and reducing supply chain lead times. Excelling on each of these dimensions removes the roadblocks to generating faster revenue growth and turning new products into profits.
In particular, competing in one of the most regulated manufacturing industries requires a quality management and compliance system that can scale across multiple production centers and supplier networks while offering support for localized regulatory requirements in each nation in which a company operates. Achieving superior product quality—from the first materials received from suppliers to delivering finished products—reduces liability and, over time, changes who a medical device manufacturer is.
How successful a medical device manufacturer will be in launching, promoting, and selling a new technology while driving and sustaining sales of existing products is entirely dependent upon quality. For lasting change to make a difference in reducing liability, product quality must become a core part of a manufacturer’s DNA.
Think of the quality management system (QMS) as the solid foundation that keeps medical device manufacturers focused on their core business. Hand-in-hand with the QMS are five strategies to help reduce liability by making quality a core part of the medical device manufacturer.
The Manufacturing Landscape—Keeping Pace with Quality Standards
Medical device manufacturers (MDMs) strive to ensure product quality and compliance are among their greatest organizational strengths. At the same time, the medical device manufacturing landscape is going through a turbulent transition as contract manufacturing organizations (CMOs) change the economics of production at a pace and level previously never seen. While MDMs welcome the opportunity to reduce production costs by partnering with CMOs, doing so brings new challenges to keeping quality levels at or above what is considered excellent from their customers’ perspectives.
The bottom line is that product quality is a prerequisite for survival for any MDM today and into the future. Quality is the life blood that keeps medical device supply chains alive today as well. Despite the rapid evolution in medical device manufacturing, McKinsey has found that by taking an aggressive strategy of improving product quality, MDMs can recover from $6 billion to $11 billion a year, according to its study, “Capturing the Value of Good Quality in Medical Devices.” McKinsey also found that major recalls and other non-routine quality events can cost a medical device manufacturer up to 11.7 percent of its market segment revenue or nearly $300 million in less than a year.
CMOs are redefining product costs and the financial structure of medical products manufacturing while customers are expecting a steady pipeline of innovative new products that meet their needs. In trying to adapt to these dual challenges, many manufacturers are seeing their quality and compliance initiatives fall short. One of the quickest ways to solve this problem is to have senior management teams champion the quality and compliance initiatives corporate-wide, further reinforcing quality as a core value of the company. CEOs have been known to increase bonuses and reward behavior that leads to higher quality with cash incentive programs. All of these efforts center on ensuring quality and compliance are maintained at a sufficient level to allow manufacturing operations to continue and keep in good standing with federal and regulatory compliance agencies.
MDMs competing in the therapeutics markets of orthopedics, cardiovascular care, and neurology are the most reliant on CMOs and have a corresponding challenge of keeping quality and compliance at superior levels. As more medical device manufacturers rely on CMOs, there will be a corresponding increase in track and traceability, quality audits, supplier quality management, and fine-tuning of supply chain networks to higher product quality standards.
Figure 1 from The Boston Consulting Group (BCG) article “Medtech Manufacturing’s Inflection Point,” underscores how quality is now the most important driver behind recent major manufacturing investments or changes. Notably, as the graphic from BCG’s article illustrates, while quality performance ranked as the top driver behind recent manufacturing investments or changes, on average, manufacturers only achieved 71 percent of the target objectives for their projects.
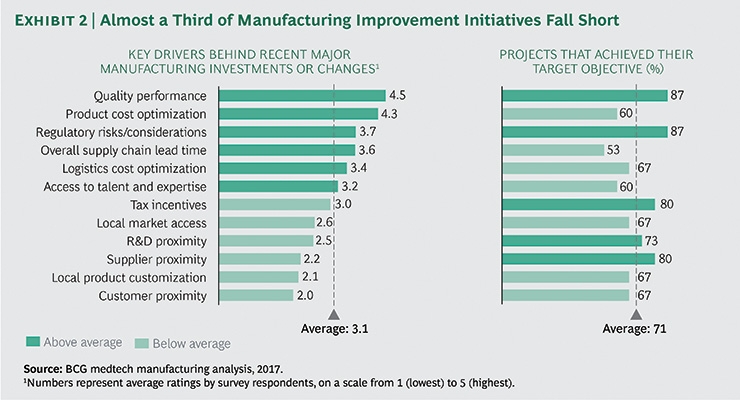
Figure 1. Image courtesy of The Boston Consulting Group.
The implication of the BCG survey was clear. As MDMs scramble to keep pace with ever-tightening product lifecycles and offload production to CMOs, too often compliance and quality management suffer even in the well-planned development and transitions of product lines.
Left unchecked, the relatively minor product and process compliance and quality problems that inevitably happen in fast-moving production businesses escalate into legal liabilities. When that happens, the nature of compliance QMS systems change from pursuing growth to protecting a company from fines and potential lawsuits from customers.
Quality and compliance initiatives fall short when there isn’t a consistent way of capturing data and turning it into intelligence and insight. That is what makes handing over medical products to a CMO so challenging. Core quality management and compliance systems tailored to an MDM’s operation don’t scale well into a CMO’s unique production operation.
Instead of having the CMO attempt to replicate the unique aspects of a current QMS, it is a better idea to start with five key strategies for reducing liability by excelling at quality.
1. Conduct Audits Based on Realistic KPIs
The starting point for gaining actionable data and intelligence on the current state of quality and compliance is to complete an internal audit of every phase of supplier, production, and service workflows. Audits are invaluable sources of data and need to be seen as one of the positive steps a manufacturer can take to improve. The most successful ones are actively endorsed and driven by the senior management team, as they have the ability to remove roadblocks and solve problems that cause quality lapses and failures.
One of the most valuable findings of audits involves seeing where quality management systems have broken down and production teams are using shortcuts or other stop-gap measures to meet tight production deadlines while performing at just-above mediocre quality levels. When it comes to complying with the U.S. Food & Drug Administration (FDA) regulations and International Standards Organization (ISO) standards, shortcuts lead to mediocrity and written warnings. It is better to use the audit to define the best possible roadmap to excellent product quality that completely mitigates the risk of liability.
Still, too often quality assurance, quality inspections, and quality control in medical product manufacturing are seen as the departments that derail getting products to market fast. This is why senior management needs to be involved, to underscore and accentuate just how critical quality is and make it a strategic priority, rather than a necessary cost or potential delay. It is time to celebrate what quality engineers and professionals bring to the medical device manufacturing process.
Audits and the key performance indicators (KPIs) they rely on need to take an integrated approach to the entire manufacturing operation, including supplier networks and distribution partners. That way, KPIs reflect the entire enterprise’s level of quality readiness, including potential areas of liability that need to be addressed.
Aggregating quality and compliance data, insights, and knowledge gained from audits requires an enterprise framework. Many MDMs have adopted the House of Quality framework specifically for this purpose. The McKinsey & Company report “Regulatory Excellence: Achieving Public Health Impact Through Distinctive Regulatory Management System” illustrates how the House of Quality is enabling MDMs to reach and exceed their quality goals based on interviews McKinsey completed with them to create their study. The House of Quality is a common framework manufacturers in highly regulated industries rely upon to orchestrate all quality management and compliance initiatives to support a common, strategic goal.
The use of audits is central to tracking KPIs and keeping quality strategies focused. Every quality strategy or initiative needs to have ongoing feedback and data on performance to stay on track. Course correcting quickly to keep on track with quality goals and objectives is essential for improvement. And selecting the best possible KPIs to fuel continuous feedback is a must-have. Audits coupled with the metrics and KPIs they rely on need to be integrated into a scalable process that can flex to the unique needs of every production operation being audited. Audits are a regular check-up on how a given production center, line, or entire series of manufacturing operations are performing. The scope and scale of quality audits vary by type of manufacturer, yet all share a common set of characteristics.
Following are the lessons learned from leading manufacturers on how to accomplish a successful quality audit.
2. Create a Single Version of Customer Requirements
It is empowering and essential to provide universal access to product and process requirements and designs to all team members in component makers, service providers, and contract manufacturers. Companies can dramatically reduce the risks of misunderstandings and mistakes by having up-to-date customer and internal documents from computer-aided design (CAD) models, drawings, images, shipping requirements, and work instructions available in a common repository as a design history record (DHR) for review prior, during, and after testing, clinical trials, and production operations.
The scope of these includes six different systems in support of the DHR to record requirements and manage customer approvals to keep all stakeholders informed.
3. Conduct Real-Time Diagnostics and Trend Analysis
Conducting real-time diagnostics and trend analysis is an effective way to find the highest performing production areas and replicate the lessons learned company-wide. The technologies enabling real-time data capture are also fueling a revolution in manufacturing quality. Programmable logic controllers (PLCs) are now commonplace across many manufacturing operations, providing up-to-the-moment data on machine quality, reliability, and performance. Machine-to-machine (M2M) technology is also bringing real-time data to statistical process control (SPC) applications capable of providing real-time alerts, graphs, and reports. And the potential for the Internet of Things to capture data that has eluded manufacturers for many years is now within reach.
Continually improving quality by using real-time data for SPC analysis provides a realistic, actionable roadmap for improvement. Knowing which production processes, machines, work centers, and product lines are operating at high-quality levels—and which are not—is essential for keeping shop floor operations running smoothly. Having immediate data to use in SPC enables continual tracking, controlling, and fine-tuning of manufacturing processes. Machine operators can also get a real-time view of process performance using quick inspection, control, and trend charts. Setting up alerts in SPC modules to signal quality management, production engineering, and scheduling when there is a deviation in performance can help avert millions of dollars in lost production time.
Essential to meeting quality goals is the ability to attain higher levels of compliance and traceability by receiving data directly from any machine on the shop floor in real time. Given the rapid advances in PLC-based monitoring and M2M interfaces, it is possible to capture up-to-the-moment data on metrics and KPIs of interest. Collecting data across the shop floor in real time and looking for patterns, trends, and predictive insights forms the foundation of manufacturing intelligence. Today, it is possible to capture the item number, manufacturing number, work order details, lot numbers, date, time, and additional KPIs to make traceability one of the strongest aspects of a manufacturing operation.
4. Rely on Tracking and Traceability to Ensure Compliance
The use of track and trace processes and systems provides medical device manufacturers with the data, insights, and information they need to stay in compliance with many of the most demanding compliance requirements in manufacturing today. ISO 13485 and 9001 standards, Current Good Manufacturing Practice (CGMP), and Code of Federal Regulations (CFR) and FDA mandates are just a few of the many compliance requirements MDMs need to meet to stay in business. Market leaders use these compliance requirements as fuel to drive even greater levels of production and process efficiency throughout their multiple plant locations.
Excelling at FDA 21 CFR Part 820 compliance while reducing manufacturing costs is the path to profitable growth for all MDMs today. However, the ability to reduce manufacturing costs, stay in compliance with 21 CFR Part 820, and gain greater accuracy and speed in supply chains is all dependent on having a proven track and traceability framework in place. Add to this the requirement of delivering the complete medical device history for each unit produced, and it is clear that track and traceability processes have a direct impact on production efficiency.
Track and traceability are invaluable for troubleshooting supplier quality problems and averting larger challenges in the future. The more production locations a manufacturer has, the more important supply chain visibility is to maintaining quality levels and meeting production forecasts. Track and traceability processes save many manufacturers from the high, unpredictable expenses of a product recall by catching product quality problems early.
5. Scale the Company QMS to Include Supplier Quality Management
Manufacturers can reduce the risk of receiving FDA warning letters and audits by relying on a quality management system to manage and scale supplier quality management and visibility as the central system of record. An enterprise-wide QMS is integrated with enterprise resource planning (ERP) and manufacturing execution system (MES) applications to gain the data needed to further analyze, find, report, and predict where product quality problems could occur. By having real-time integration links to ERP and MES applications to gain access to quality-related data, the QMS serves as the system of record for all quality-based activity. Because it is common for internal auditors to find discrepancies in how data is transferred across ERP, MES, and QMS solutions, many are using application programming interfaces and web services to bridge the gap between disparate systems, reduce data latency, and increase data quality.
Once an integration strategy is in place that delivers accurate, reliable data from ERP and MES solutions, manufacturers often expand to include supplier quality management (SQM) modules. An SQM module extends the tracking and traceability often multiple levels deep into a supplier network, provides advanced reporting on supplier quality yields, and has predefined workflows for completing supplier audits. SQM modules can also streamline supplier onboarding while assigning quality and compliance goals to them and baselining their performance with every shipment. An SQM addition to a QMS suite is a must-have for MDMs relying on diverse supplier networks that are continually being modified to support new product development.
In addition, the most effective strategies medical device manufacturers are using today to mitigate risk involve supply chain planning, forecasting, and ongoing supplier quality benchmarking, including defining minimum quality standards over time. All of these benefits are available from SQM modules and their related options. By relying on a QMS that is integrated with ERP, MES, and SQM systems as a platform for creating greater levels of supplier collaboration, medical device manufacturers can define more agile, responsive frameworks that flex with customer requirements.
By starting with the mindset of suppliers as collaborators, manufacturers are better positioned to build frameworks that mitigate or reduce supply chain risk while fueling innovation. From providing customized new parts that accelerate new product development schedules to stepping up quality levels on long-standing components, when suppliers become collaborators in creation, risk mitigation strategies improve.
Louis Columbus is a principal at manufacturing ERP provider IQMS. He is also a contributing writer and analyst for Forbes. Previous positions include director product management at Ingram Cloud, vice president of marketing at iBASEt and Plex Systems, senior analyst at AMR Research (now Gartner), and marketing and business development at SaaS startups. His academic background includes an MBA from Pepperdine University and the Strategic Marketing Management and Digital Marketing Programs at Stanford University Graduate School of Business. Columbus also teaches MBA courses in international business, global competitive strategies, international market research, strategic planning, and market research. He is currently a member of the faculty at Webster University and has taught at California State University, Fullerton; University of California, Irvine; and Marymount University. He has authored 16 books on a variety of IT-related technologies.
Bibliography
In particular, competing in one of the most regulated manufacturing industries requires a quality management and compliance system that can scale across multiple production centers and supplier networks while offering support for localized regulatory requirements in each nation in which a company operates. Achieving superior product quality—from the first materials received from suppliers to delivering finished products—reduces liability and, over time, changes who a medical device manufacturer is.
How successful a medical device manufacturer will be in launching, promoting, and selling a new technology while driving and sustaining sales of existing products is entirely dependent upon quality. For lasting change to make a difference in reducing liability, product quality must become a core part of a manufacturer’s DNA.
Think of the quality management system (QMS) as the solid foundation that keeps medical device manufacturers focused on their core business. Hand-in-hand with the QMS are five strategies to help reduce liability by making quality a core part of the medical device manufacturer.
The Manufacturing Landscape—Keeping Pace with Quality Standards
Medical device manufacturers (MDMs) strive to ensure product quality and compliance are among their greatest organizational strengths. At the same time, the medical device manufacturing landscape is going through a turbulent transition as contract manufacturing organizations (CMOs) change the economics of production at a pace and level previously never seen. While MDMs welcome the opportunity to reduce production costs by partnering with CMOs, doing so brings new challenges to keeping quality levels at or above what is considered excellent from their customers’ perspectives.
The bottom line is that product quality is a prerequisite for survival for any MDM today and into the future. Quality is the life blood that keeps medical device supply chains alive today as well. Despite the rapid evolution in medical device manufacturing, McKinsey has found that by taking an aggressive strategy of improving product quality, MDMs can recover from $6 billion to $11 billion a year, according to its study, “Capturing the Value of Good Quality in Medical Devices.” McKinsey also found that major recalls and other non-routine quality events can cost a medical device manufacturer up to 11.7 percent of its market segment revenue or nearly $300 million in less than a year.
CMOs are redefining product costs and the financial structure of medical products manufacturing while customers are expecting a steady pipeline of innovative new products that meet their needs. In trying to adapt to these dual challenges, many manufacturers are seeing their quality and compliance initiatives fall short. One of the quickest ways to solve this problem is to have senior management teams champion the quality and compliance initiatives corporate-wide, further reinforcing quality as a core value of the company. CEOs have been known to increase bonuses and reward behavior that leads to higher quality with cash incentive programs. All of these efforts center on ensuring quality and compliance are maintained at a sufficient level to allow manufacturing operations to continue and keep in good standing with federal and regulatory compliance agencies.
MDMs competing in the therapeutics markets of orthopedics, cardiovascular care, and neurology are the most reliant on CMOs and have a corresponding challenge of keeping quality and compliance at superior levels. As more medical device manufacturers rely on CMOs, there will be a corresponding increase in track and traceability, quality audits, supplier quality management, and fine-tuning of supply chain networks to higher product quality standards.
Figure 1 from The Boston Consulting Group (BCG) article “Medtech Manufacturing’s Inflection Point,” underscores how quality is now the most important driver behind recent major manufacturing investments or changes. Notably, as the graphic from BCG’s article illustrates, while quality performance ranked as the top driver behind recent manufacturing investments or changes, on average, manufacturers only achieved 71 percent of the target objectives for their projects.

Figure 1. Image courtesy of The Boston Consulting Group.
The implication of the BCG survey was clear. As MDMs scramble to keep pace with ever-tightening product lifecycles and offload production to CMOs, too often compliance and quality management suffer even in the well-planned development and transitions of product lines.
Left unchecked, the relatively minor product and process compliance and quality problems that inevitably happen in fast-moving production businesses escalate into legal liabilities. When that happens, the nature of compliance QMS systems change from pursuing growth to protecting a company from fines and potential lawsuits from customers.
Quality and compliance initiatives fall short when there isn’t a consistent way of capturing data and turning it into intelligence and insight. That is what makes handing over medical products to a CMO so challenging. Core quality management and compliance systems tailored to an MDM’s operation don’t scale well into a CMO’s unique production operation.
Instead of having the CMO attempt to replicate the unique aspects of a current QMS, it is a better idea to start with five key strategies for reducing liability by excelling at quality.
1. Conduct Audits Based on Realistic KPIs
The starting point for gaining actionable data and intelligence on the current state of quality and compliance is to complete an internal audit of every phase of supplier, production, and service workflows. Audits are invaluable sources of data and need to be seen as one of the positive steps a manufacturer can take to improve. The most successful ones are actively endorsed and driven by the senior management team, as they have the ability to remove roadblocks and solve problems that cause quality lapses and failures.
One of the most valuable findings of audits involves seeing where quality management systems have broken down and production teams are using shortcuts or other stop-gap measures to meet tight production deadlines while performing at just-above mediocre quality levels. When it comes to complying with the U.S. Food & Drug Administration (FDA) regulations and International Standards Organization (ISO) standards, shortcuts lead to mediocrity and written warnings. It is better to use the audit to define the best possible roadmap to excellent product quality that completely mitigates the risk of liability.
Still, too often quality assurance, quality inspections, and quality control in medical product manufacturing are seen as the departments that derail getting products to market fast. This is why senior management needs to be involved, to underscore and accentuate just how critical quality is and make it a strategic priority, rather than a necessary cost or potential delay. It is time to celebrate what quality engineers and professionals bring to the medical device manufacturing process.
Audits and the key performance indicators (KPIs) they rely on need to take an integrated approach to the entire manufacturing operation, including supplier networks and distribution partners. That way, KPIs reflect the entire enterprise’s level of quality readiness, including potential areas of liability that need to be addressed.
Aggregating quality and compliance data, insights, and knowledge gained from audits requires an enterprise framework. Many MDMs have adopted the House of Quality framework specifically for this purpose. The McKinsey & Company report “Regulatory Excellence: Achieving Public Health Impact Through Distinctive Regulatory Management System” illustrates how the House of Quality is enabling MDMs to reach and exceed their quality goals based on interviews McKinsey completed with them to create their study. The House of Quality is a common framework manufacturers in highly regulated industries rely upon to orchestrate all quality management and compliance initiatives to support a common, strategic goal.
The use of audits is central to tracking KPIs and keeping quality strategies focused. Every quality strategy or initiative needs to have ongoing feedback and data on performance to stay on track. Course correcting quickly to keep on track with quality goals and objectives is essential for improvement. And selecting the best possible KPIs to fuel continuous feedback is a must-have. Audits coupled with the metrics and KPIs they rely on need to be integrated into a scalable process that can flex to the unique needs of every production operation being audited. Audits are a regular check-up on how a given production center, line, or entire series of manufacturing operations are performing. The scope and scale of quality audits vary by type of manufacturer, yet all share a common set of characteristics.
Following are the lessons learned from leading manufacturers on how to accomplish a successful quality audit.
- Capture data on process and product areas that keep the focus of the audit on customers and their outcomes first.
- Define the audit goals, timing, and type based on the data obtained from customers and company-wide.
- Select a cross functionally based trained audit team, and appoint a senior executive as champion.
- Define audit modules, and assign auditors to complete each according to the overall audit project plan.
- Perform the plant audit on a module-by-module basis, designing the cadence and timeframes to meet ISO requirements.
- Summarize the findings, and propose a plan of action for solving the quality problems immediately.
- Look for new metrics and KPIs that can emerge from audits and provide insights into a new area of process improvement and manufacturing quality.
- Use Six Sigma and DMAIC (Define, Measure, Analyze, Improve, Control) based data to drive manufacturing intelligence and predictive analytics of where and how quality can be improved.
2. Create a Single Version of Customer Requirements
It is empowering and essential to provide universal access to product and process requirements and designs to all team members in component makers, service providers, and contract manufacturers. Companies can dramatically reduce the risks of misunderstandings and mistakes by having up-to-date customer and internal documents from computer-aided design (CAD) models, drawings, images, shipping requirements, and work instructions available in a common repository as a design history record (DHR) for review prior, during, and after testing, clinical trials, and production operations.
The scope of these includes six different systems in support of the DHR to record requirements and manage customer approvals to keep all stakeholders informed.
- Document control system with version change control
- Product lifecycle management with links to CAD systems
- Audit management for plans and results of internal and third-party audits
- CAPA reports and issues resolution system
- Testing management to capture records of internal tests and results from clinical trials
- An overarching automated workflow system to drive electronic review, approvals, and issues closure for all of these records
3. Conduct Real-Time Diagnostics and Trend Analysis
Conducting real-time diagnostics and trend analysis is an effective way to find the highest performing production areas and replicate the lessons learned company-wide. The technologies enabling real-time data capture are also fueling a revolution in manufacturing quality. Programmable logic controllers (PLCs) are now commonplace across many manufacturing operations, providing up-to-the-moment data on machine quality, reliability, and performance. Machine-to-machine (M2M) technology is also bringing real-time data to statistical process control (SPC) applications capable of providing real-time alerts, graphs, and reports. And the potential for the Internet of Things to capture data that has eluded manufacturers for many years is now within reach.
Continually improving quality by using real-time data for SPC analysis provides a realistic, actionable roadmap for improvement. Knowing which production processes, machines, work centers, and product lines are operating at high-quality levels—and which are not—is essential for keeping shop floor operations running smoothly. Having immediate data to use in SPC enables continual tracking, controlling, and fine-tuning of manufacturing processes. Machine operators can also get a real-time view of process performance using quick inspection, control, and trend charts. Setting up alerts in SPC modules to signal quality management, production engineering, and scheduling when there is a deviation in performance can help avert millions of dollars in lost production time.
Essential to meeting quality goals is the ability to attain higher levels of compliance and traceability by receiving data directly from any machine on the shop floor in real time. Given the rapid advances in PLC-based monitoring and M2M interfaces, it is possible to capture up-to-the-moment data on metrics and KPIs of interest. Collecting data across the shop floor in real time and looking for patterns, trends, and predictive insights forms the foundation of manufacturing intelligence. Today, it is possible to capture the item number, manufacturing number, work order details, lot numbers, date, time, and additional KPIs to make traceability one of the strongest aspects of a manufacturing operation.
4. Rely on Tracking and Traceability to Ensure Compliance
The use of track and trace processes and systems provides medical device manufacturers with the data, insights, and information they need to stay in compliance with many of the most demanding compliance requirements in manufacturing today. ISO 13485 and 9001 standards, Current Good Manufacturing Practice (CGMP), and Code of Federal Regulations (CFR) and FDA mandates are just a few of the many compliance requirements MDMs need to meet to stay in business. Market leaders use these compliance requirements as fuel to drive even greater levels of production and process efficiency throughout their multiple plant locations.
Excelling at FDA 21 CFR Part 820 compliance while reducing manufacturing costs is the path to profitable growth for all MDMs today. However, the ability to reduce manufacturing costs, stay in compliance with 21 CFR Part 820, and gain greater accuracy and speed in supply chains is all dependent on having a proven track and traceability framework in place. Add to this the requirement of delivering the complete medical device history for each unit produced, and it is clear that track and traceability processes have a direct impact on production efficiency.
Track and traceability are invaluable for troubleshooting supplier quality problems and averting larger challenges in the future. The more production locations a manufacturer has, the more important supply chain visibility is to maintaining quality levels and meeting production forecasts. Track and traceability processes save many manufacturers from the high, unpredictable expenses of a product recall by catching product quality problems early.
5. Scale the Company QMS to Include Supplier Quality Management
Manufacturers can reduce the risk of receiving FDA warning letters and audits by relying on a quality management system to manage and scale supplier quality management and visibility as the central system of record. An enterprise-wide QMS is integrated with enterprise resource planning (ERP) and manufacturing execution system (MES) applications to gain the data needed to further analyze, find, report, and predict where product quality problems could occur. By having real-time integration links to ERP and MES applications to gain access to quality-related data, the QMS serves as the system of record for all quality-based activity. Because it is common for internal auditors to find discrepancies in how data is transferred across ERP, MES, and QMS solutions, many are using application programming interfaces and web services to bridge the gap between disparate systems, reduce data latency, and increase data quality.
Once an integration strategy is in place that delivers accurate, reliable data from ERP and MES solutions, manufacturers often expand to include supplier quality management (SQM) modules. An SQM module extends the tracking and traceability often multiple levels deep into a supplier network, provides advanced reporting on supplier quality yields, and has predefined workflows for completing supplier audits. SQM modules can also streamline supplier onboarding while assigning quality and compliance goals to them and baselining their performance with every shipment. An SQM addition to a QMS suite is a must-have for MDMs relying on diverse supplier networks that are continually being modified to support new product development.
In addition, the most effective strategies medical device manufacturers are using today to mitigate risk involve supply chain planning, forecasting, and ongoing supplier quality benchmarking, including defining minimum quality standards over time. All of these benefits are available from SQM modules and their related options. By relying on a QMS that is integrated with ERP, MES, and SQM systems as a platform for creating greater levels of supplier collaboration, medical device manufacturers can define more agile, responsive frameworks that flex with customer requirements.
By starting with the mindset of suppliers as collaborators, manufacturers are better positioned to build frameworks that mitigate or reduce supply chain risk while fueling innovation. From providing customized new parts that accelerate new product development schedules to stepping up quality levels on long-standing components, when suppliers become collaborators in creation, risk mitigation strategies improve.
Louis Columbus is a principal at manufacturing ERP provider IQMS. He is also a contributing writer and analyst for Forbes. Previous positions include director product management at Ingram Cloud, vice president of marketing at iBASEt and Plex Systems, senior analyst at AMR Research (now Gartner), and marketing and business development at SaaS startups. His academic background includes an MBA from Pepperdine University and the Strategic Marketing Management and Digital Marketing Programs at Stanford University Graduate School of Business. Columbus also teaches MBA courses in international business, global competitive strategies, international market research, strategic planning, and market research. He is currently a member of the faculty at Webster University and has taught at California State University, Fullerton; University of California, Irvine; and Marymount University. He has authored 16 books on a variety of IT-related technologies.
Bibliography
- Boston Consulting Group (2017, Dec. 6) Medtech Manufacturing’s Inflection Point. Michele Brocca, Kevin D’Souza, Ted Sisko and Samantha Baron. Retrieved from the Boston Consulting Group site: http://bit.ly/mpo180320
- Chira, R., & Musetescu, A. (2016). The Impact of Customer Service On Logistics. Revista Economica, 68(3), 24-31.
- Dyer, J. H., & Nobeoka, K. (2000). Creating and Managing A High-Performance Knowledge-Sharing Network: The Toyota Case. Strategic Management Journal, 21(3), 345.
- Fuhr, T., Silverman, S., Telpis, V., & Makarova, E. (2017, February). Capturing the Value of Good Quality in Medical Devices. Retrieved from: http://bit.ly/mpo180321
- Fuhr, T., George, K., Pai, Janice (2013) The Business Case for Medical Device Quality, McKinsey Center for Government. Retrieved from: http://bit.ly/mpo180322
- Garga, E., & Bambale, A. J. A. (2016). The Impact of Service Quality on Customer Patronage: Mediating Effects of Switching Cost and Customer Satisfaction. International Journal of Global Business, 9(1).
- Kaur, P. (2009). Current Cost of Quality Management Practices in India in the Era of Globalization: An Empirical Study of Selected Companies. Decision (0304-0941), 36(1).
- Novack, R. A., & Thomas, D. J. (2004). The Challenges of Implementing the Perfect Order Concept. Transportation Journal (American Society Of Transportation & Logistics Inc), 43(1), 5-16.
- Youngkyo, S. (2016). Mother Factory vs. Model Factory: Comparative Study of International Knowledge Transfer. Annals of Business Administrative Science, 15(6), 251-263.




























