David R. Somers, Senior Industry Analyst 08.05.16
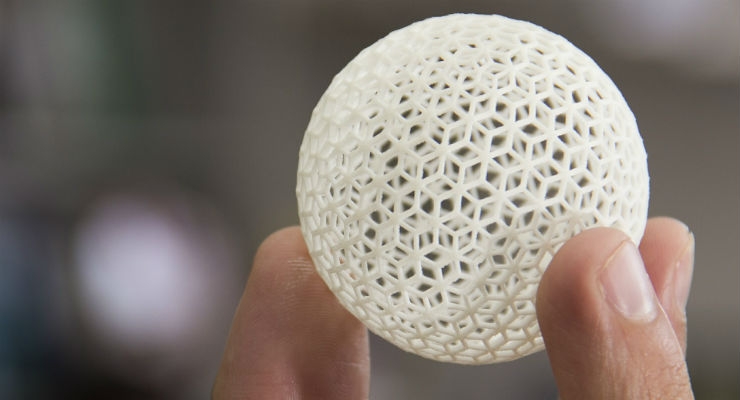
Not since the development of electron beam (e-beam) evaporation and thin layer epitaxy for silicon wafer manufacturing has the industry of small-scale (down to the nano-substrate level) applied technology been so exciting for the development of products that, until now, were very difficult to even produce, much less improve. The technology I refer to is that of 3D printing.
The FDA is seeking to understand the applicability of 3D printing for medical devices. Their recent offering on July 14th made a presentation through a Grand Round webcast as part of a larger program sponsored by the U.S. Navy and FDA to present a frame of reference and thinking behind the effects of such technology within the context of medical devices.
This article seeks to present the essence of that presentation within an historical context leading up to the latest in technological innovation and where the regulatory perspective fits in with these new developments.
Historical Context
As mentioned previously, the e-beam evaporation was one of a source material being evaporated onto a substrate; the other was thin layer epitaxy constructing a thin "epitaxial" layer of single-crystal material deposited on single-crystal substrate to grow a crystallographic structure. Industry is exploring a variety of manufacturing techniques and technologies creating potential physical objects that can enable small scale (and volume) of manufacturing processes, develop a unique and personalized object based on computer graphic specifications, and incorporate a wide variety of materials (e.g., plastics, metals, carbon-based high density polymers).
With 3D printing, the technique can involve both the additive method (building layer upon layer to form an object), as well as the reductive method (elimination of material from a material blank) with a pre-programmed outcome, as a sculptor on marble or wood.
First Inklings
Several years ago, my niece and I took a trip to New York City to see the displays of some exotic art and contemporaneous designs coming out of the artistic community, using some radical technologies that were completely bespoke and prototypical for the outputs displayed. Only the example of a “Velociraptor larynx and voice box” were known to the general public from the second Speilberg classic, “Jurassic Park” as having been “printed” on a device incorporating a computer-aided design and some wax material being deposited around a matrix to form the object. It enthralled many and piqued much interest in the manufacturing and medical device community at the time—imagine “printing” human parts and other organelles by individual design, custom fit to patients needing lifesaving products to preserve and improve the quality of life for many.
As she and I were impressed, there weren’t many direct and tangible applications visible to the general public at that time—but that was changing more rapidly than we could ever imagine.
Today’s Regulatory Environment
The FDA’s Grand Rounds webcast on July 14th offered the latest in applied technology and, to a limited extent, the impact of regulatory guidance affecting that development to ensure both safety and efficacy are preserved. Lt. James Coburn, a senior researcher at the FDA’s Center for Devices and Radiological Health (CDRH) and a member of the Additive Manufacturing Working Group, began his presentation with an overview of the main focus of their efforts at enabling manufacturers to make new designs within the areas of medical implants and tissue engineering, as well as facilitating combination drugs and devices that are personalized. Both the internal resources of FDA’s regulatory science and engineering, working in concert with commercial and institutional scientists, can benefit from 3D-print technology. As a result, the concept of cooperation is gaining rapid acceptance as the standard in specialized, patient-specific manufacturing. This effort is in process on several products that are currently working their way through their existing regulatory pathways, with more research in the future.
Coburn stated that, “We want to give guidance and clarity through research, interactions, and colaborations.” Associated with that are cogent and well-defined metrics and tools enabling data collection for processing through the federal regulatory approval system to get things to clinicians and patients faster. A public partnership with the National Manufacturing Innovation Institutes has combined the resources of leaders in the healthcare profession, government, and educational institutions, resulting in a projected growth of 3D printing in medical devices by 2025. From a regulatory standpoint, the FDA is currently analyzing [PDF] the applicability of current laws to those devices produced by the new techniques, including the design control and data security issues surrounding code.
The Future Is Today
The seminar provided an overview on the various applied techniques currently in use or anticipated, including extrusion, materials jetting, laser sintering (SLS) melting of metals and alloys, stereo lithography, binder jetting, laser transfer, and fused deposition (FDM).
Of note was the biodegradeable tissue scaffolding providing a matrix enabling growing materials to facilitate the replication of original objects matching their parameters to the actual structure.
Given the emphasis on the emerging techology, industry leaders in manufacturing software for medical devices have addressed this capability. During the QAD Explore Medical Device executive roundtable in May of this year, the topic emerged as a significant concern among both providers of 3D manufactured products, as well as those who govern the enabling technologies of 3D production products. For example, in 2010, FDA approved the InteGrip, an acetabular cup manufactured by Exactech with a titanium alloy incorporating a porous surface manufactured using 3D printing.
Conclusion
3D-printed medical devices are not only the future…that future is now. The technology has been, and will be, making significant impacts on products and services for not only the medical device industry, but around the world as well. Already products for jet engine parts for compression fuel nozzles, mini hearing aids, and others are in active use. The FDA and industry are an integral part of that effort.
Within the regulatory world, the time frame for comments submitted by all interested parties to FDA for regulatory considerations for this new technology ends August 8, 2016. This technology is a game changer for manufacturers and those who develop products in support of those products. By the collaborative efforts of both government and industry enabling the technology and making sure they meet the stringent safety and efficacy standards, all will benefit…especially the patients using these products.
David R. Somers is a senior industry analyst with Axendia Inc., a leading trusted advisor to the life-science and healthcare industries. The company provides trusted counsel to industry stakeholders on business, regulatory and technology issues.
The FDA is seeking to understand the applicability of 3D printing for medical devices. Their recent offering on July 14th made a presentation through a Grand Round webcast as part of a larger program sponsored by the U.S. Navy and FDA to present a frame of reference and thinking behind the effects of such technology within the context of medical devices.
This article seeks to present the essence of that presentation within an historical context leading up to the latest in technological innovation and where the regulatory perspective fits in with these new developments.
Historical Context
As mentioned previously, the e-beam evaporation was one of a source material being evaporated onto a substrate; the other was thin layer epitaxy constructing a thin "epitaxial" layer of single-crystal material deposited on single-crystal substrate to grow a crystallographic structure. Industry is exploring a variety of manufacturing techniques and technologies creating potential physical objects that can enable small scale (and volume) of manufacturing processes, develop a unique and personalized object based on computer graphic specifications, and incorporate a wide variety of materials (e.g., plastics, metals, carbon-based high density polymers).
With 3D printing, the technique can involve both the additive method (building layer upon layer to form an object), as well as the reductive method (elimination of material from a material blank) with a pre-programmed outcome, as a sculptor on marble or wood.
First Inklings
Several years ago, my niece and I took a trip to New York City to see the displays of some exotic art and contemporaneous designs coming out of the artistic community, using some radical technologies that were completely bespoke and prototypical for the outputs displayed. Only the example of a “Velociraptor larynx and voice box” were known to the general public from the second Speilberg classic, “Jurassic Park” as having been “printed” on a device incorporating a computer-aided design and some wax material being deposited around a matrix to form the object. It enthralled many and piqued much interest in the manufacturing and medical device community at the time—imagine “printing” human parts and other organelles by individual design, custom fit to patients needing lifesaving products to preserve and improve the quality of life for many.
As she and I were impressed, there weren’t many direct and tangible applications visible to the general public at that time—but that was changing more rapidly than we could ever imagine.
Today’s Regulatory Environment
The FDA’s Grand Rounds webcast on July 14th offered the latest in applied technology and, to a limited extent, the impact of regulatory guidance affecting that development to ensure both safety and efficacy are preserved. Lt. James Coburn, a senior researcher at the FDA’s Center for Devices and Radiological Health (CDRH) and a member of the Additive Manufacturing Working Group, began his presentation with an overview of the main focus of their efforts at enabling manufacturers to make new designs within the areas of medical implants and tissue engineering, as well as facilitating combination drugs and devices that are personalized. Both the internal resources of FDA’s regulatory science and engineering, working in concert with commercial and institutional scientists, can benefit from 3D-print technology. As a result, the concept of cooperation is gaining rapid acceptance as the standard in specialized, patient-specific manufacturing. This effort is in process on several products that are currently working their way through their existing regulatory pathways, with more research in the future.
Coburn stated that, “We want to give guidance and clarity through research, interactions, and colaborations.” Associated with that are cogent and well-defined metrics and tools enabling data collection for processing through the federal regulatory approval system to get things to clinicians and patients faster. A public partnership with the National Manufacturing Innovation Institutes has combined the resources of leaders in the healthcare profession, government, and educational institutions, resulting in a projected growth of 3D printing in medical devices by 2025. From a regulatory standpoint, the FDA is currently analyzing [PDF] the applicability of current laws to those devices produced by the new techniques, including the design control and data security issues surrounding code.
The Future Is Today
The seminar provided an overview on the various applied techniques currently in use or anticipated, including extrusion, materials jetting, laser sintering (SLS) melting of metals and alloys, stereo lithography, binder jetting, laser transfer, and fused deposition (FDM).
Of note was the biodegradeable tissue scaffolding providing a matrix enabling growing materials to facilitate the replication of original objects matching their parameters to the actual structure.
Given the emphasis on the emerging techology, industry leaders in manufacturing software for medical devices have addressed this capability. During the QAD Explore Medical Device executive roundtable in May of this year, the topic emerged as a significant concern among both providers of 3D manufactured products, as well as those who govern the enabling technologies of 3D production products. For example, in 2010, FDA approved the InteGrip, an acetabular cup manufactured by Exactech with a titanium alloy incorporating a porous surface manufactured using 3D printing.
Conclusion
3D-printed medical devices are not only the future…that future is now. The technology has been, and will be, making significant impacts on products and services for not only the medical device industry, but around the world as well. Already products for jet engine parts for compression fuel nozzles, mini hearing aids, and others are in active use. The FDA and industry are an integral part of that effort.
Within the regulatory world, the time frame for comments submitted by all interested parties to FDA for regulatory considerations for this new technology ends August 8, 2016. This technology is a game changer for manufacturers and those who develop products in support of those products. By the collaborative efforts of both government and industry enabling the technology and making sure they meet the stringent safety and efficacy standards, all will benefit…especially the patients using these products.
David R. Somers is a senior industry analyst with Axendia Inc., a leading trusted advisor to the life-science and healthcare industries. The company provides trusted counsel to industry stakeholders on business, regulatory and technology issues.