GlobeNewswire08.24.20
Guardant Health Inc. announces the U.S. Food and Drug Administration (FDA) has granted the Guardant-19 test emergency use authorization (EUA) for use in the detection of the novel coronavirus, SARS-CoV-2. The test is being offered to Guardant Health employees and select partner organizations through the company’s CLIA-certified clinical laboratory.
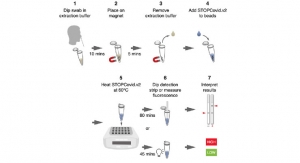
The Guardant-19 test is a reverse transcriptase polymerase chain reaction next generation sequencing (rt-PCR-seq) test that detects coronavirus SARS-CoV-2 nucleic acid from upper respiratory nasal specimens including nasopharyngeal swabs, oropharyngeal swabs, nasal swabs, interior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal wash/aspirates, nasal aspirates, and nasal washes. The test has a validated limit of detection (LoD) of 125 copies per mL and results are typically returned the next day. The heavily multiplexed testing workflow used has the ability to scale to over 10,000 tests per day.
“While serving cancer patients remains our top priority, we are proud to be able to leverage our expertise in liquid biopsy testing to contribute to battling the COVID-19 pandemic by offering a highly accurate test that is truly additive to the testing options available today,” said AmirAli Talasaz, Guardant Health president. “Since the beginning of the pandemic we believed it was our social responsibility to not only protect the health and safety of our employees, but to also help our greater community with return to work and school initiatives. It gives me great pride knowing that Guardant Health is able to deliver.”
The Guardant-19 test is being used to help Delaware State University, a Historically Black College & University, in its efforts to reopen safely. “Guardant is providing us with an innovative testing technology to help protect the safety of our entire campus community,” said Tony Allen, president of Delaware State University, which is being advised by nonprofit Testing for America on its reopening plans.
“Our mission is to permanently and safely reopen schools, business and the US economy by providing affordable, accessible and frequent testing and screening. We believe that a testing option like the one provided by Guardant Health can help achieve the highly accurate and rapid results at a scale that we need,” said Dr. Joan Coker, surgeon and Advisory Council member of Testing for America.
The Healing Grove Health Center in San Jose, California is another partner organization. “We are thankful for a high-throughput, fast, accurate COVID-19 test from Guardant Health,” said Brett Bymaster, the center’s executive director. “Our patients are low-income and high risk, and we are seeing a high positivity rate. When we catch these positive cases early, we are possibly saving hundreds of people from getting infected with COVID-19 by ensuring that they quarantine. By working closely with Guardant Health, we have gotten results quickly and have been able to keep our COVID-positive patients recovering at home, limiting the severity of the outbreak in this important community.”
The Guardant-19 test is a reverse transcriptase polymerase chain reaction next generation sequencing (rt-PCR-seq) test that detects coronavirus SARS-CoV-2 nucleic acid from upper respiratory nasal specimens including nasopharyngeal swabs, oropharyngeal swabs, nasal swabs, interior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal wash/aspirates, nasal aspirates, and nasal washes. The test has a validated limit of detection (LoD) of 125 copies per mL and results are typically returned the next day. The heavily multiplexed testing workflow used has the ability to scale to over 10,000 tests per day.
“While serving cancer patients remains our top priority, we are proud to be able to leverage our expertise in liquid biopsy testing to contribute to battling the COVID-19 pandemic by offering a highly accurate test that is truly additive to the testing options available today,” said AmirAli Talasaz, Guardant Health president. “Since the beginning of the pandemic we believed it was our social responsibility to not only protect the health and safety of our employees, but to also help our greater community with return to work and school initiatives. It gives me great pride knowing that Guardant Health is able to deliver.”
The Guardant-19 test is being used to help Delaware State University, a Historically Black College & University, in its efforts to reopen safely. “Guardant is providing us with an innovative testing technology to help protect the safety of our entire campus community,” said Tony Allen, president of Delaware State University, which is being advised by nonprofit Testing for America on its reopening plans.
“Our mission is to permanently and safely reopen schools, business and the US economy by providing affordable, accessible and frequent testing and screening. We believe that a testing option like the one provided by Guardant Health can help achieve the highly accurate and rapid results at a scale that we need,” said Dr. Joan Coker, surgeon and Advisory Council member of Testing for America.
The Healing Grove Health Center in San Jose, California is another partner organization. “We are thankful for a high-throughput, fast, accurate COVID-19 test from Guardant Health,” said Brett Bymaster, the center’s executive director. “Our patients are low-income and high risk, and we are seeing a high positivity rate. When we catch these positive cases early, we are possibly saving hundreds of people from getting infected with COVID-19 by ensuring that they quarantine. By working closely with Guardant Health, we have gotten results quickly and have been able to keep our COVID-positive patients recovering at home, limiting the severity of the outbreak in this important community.”