Business Wire05.13.20
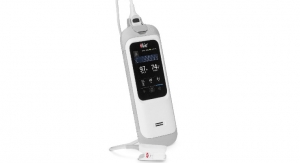
Eko, a digital health company building AI-powered screening and telehealth solutions to fight cardiovascular disease, today announced that the U.S. Food and Drug Administration (FDA) has issued the company an Emergency Use Authorization (EUA) for its novel ECG-based algorithm that can provide an easily accessible, rapid screening test for low ejection fraction (low EF), a weak heart pump.
Awarded an FDA Breakthrough Device designation in December 2019, the algorithm’s regulatory review has been further accelerated due to its potential to assist providers in identifying patients with abnormal heart function during the COVID-19 pandemic.
It is known that patients with cardiovascular disease have a much greater risk of death from COVID-19 than those with normal hearts. Patients with pre-existing cardiovascular disease are estimated by the American College of Cardiology to have a 10.5 percent case fatality rate due to COVID-19, significantly higher than those without such conditions. COVID-19 infection may also directly cause heart muscle weakness. The Emergency Use Authorization allows healthcare providers to use Eko’s AI algorithm to analyze a standard 12-lead ECG, an easily accessible and painless test to assess the risk of a weakened heart pump.
“Early detection of heart failure patients can better inform treatments for these individuals at higher risk for severe illness from COVID-19,” said Connor Landgraf, CEO and co-founder, Eko. “With the FDA’s Emergency Use Authorization, Eko will be able to offer its low EF screening at Mayo Clinic immediately, the first step in expanding the offering to other providers on the Eko platform.”
Heart failure affects about 5.7 million people in the U.S. alone and is most commonly detected by echocardiogram, a test that is not normally conducted during a physical exam, requires specially trained technicians to record, and requires prolonged contact with the patient. Because of limited access to echocardiography, especially during the COVID-19 pandemic, and because many patients with reduced heart function do not have symptoms, or have symptoms that are attributed to COVID-19, heart failure is frequently diagnosed late, making life-prolonging treatment more challenging.
“Given the danger COVID-19 poses to patients with a weak heart pump, it’s important that we rapidly identify these individuals early and monitor them closely. By embedding the heart failure screening AI into a quick, widely available, and safe test using existing medical devices, we can detect heart failure early and start appropriate treatments,” said Dr. Paul Friedman, Chair of the Department of Cardiovascular Medicine, Mayo Clinic. “Additionally, for people with COVID-19, we may be able to identify when the virus causes the development of a weak heart pump quickly, safely, and easily using these AI tools.”
Awarded an FDA Breakthrough Device designation in December 2019, the algorithm’s regulatory review has been further accelerated due to its potential to assist providers in identifying patients with abnormal heart function during the COVID-19 pandemic.
It is known that patients with cardiovascular disease have a much greater risk of death from COVID-19 than those with normal hearts. Patients with pre-existing cardiovascular disease are estimated by the American College of Cardiology to have a 10.5 percent case fatality rate due to COVID-19, significantly higher than those without such conditions. COVID-19 infection may also directly cause heart muscle weakness. The Emergency Use Authorization allows healthcare providers to use Eko’s AI algorithm to analyze a standard 12-lead ECG, an easily accessible and painless test to assess the risk of a weakened heart pump.
“Early detection of heart failure patients can better inform treatments for these individuals at higher risk for severe illness from COVID-19,” said Connor Landgraf, CEO and co-founder, Eko. “With the FDA’s Emergency Use Authorization, Eko will be able to offer its low EF screening at Mayo Clinic immediately, the first step in expanding the offering to other providers on the Eko platform.”
Heart failure affects about 5.7 million people in the U.S. alone and is most commonly detected by echocardiogram, a test that is not normally conducted during a physical exam, requires specially trained technicians to record, and requires prolonged contact with the patient. Because of limited access to echocardiography, especially during the COVID-19 pandemic, and because many patients with reduced heart function do not have symptoms, or have symptoms that are attributed to COVID-19, heart failure is frequently diagnosed late, making life-prolonging treatment more challenging.
“Given the danger COVID-19 poses to patients with a weak heart pump, it’s important that we rapidly identify these individuals early and monitor them closely. By embedding the heart failure screening AI into a quick, widely available, and safe test using existing medical devices, we can detect heart failure early and start appropriate treatments,” said Dr. Paul Friedman, Chair of the Department of Cardiovascular Medicine, Mayo Clinic. “Additionally, for people with COVID-19, we may be able to identify when the virus causes the development of a weak heart pump quickly, safely, and easily using these AI tools.”