PR Newswire02.28.20
Only 42 percent of people living with Type 1 diabetes count carbohydrates to determine their mealtime insulin dose according to the 2019 Seagrove Partners' Patients Perspectives Report. And it is generally accepted that anyone living with insulin-dependent diabetes knows the frustrations of getting carb counting wrong—along with the so-called rollercoaster of hypo- and hyperglycemia that ensues. Considering that all FDA cleared dose calculators have thus far required the user to do carb counting themselves, it is fair to say that the majority of dose calculators have been designed for the minority of people living with Type 1 and Type 2 diabetes. But this is a thing of the past.
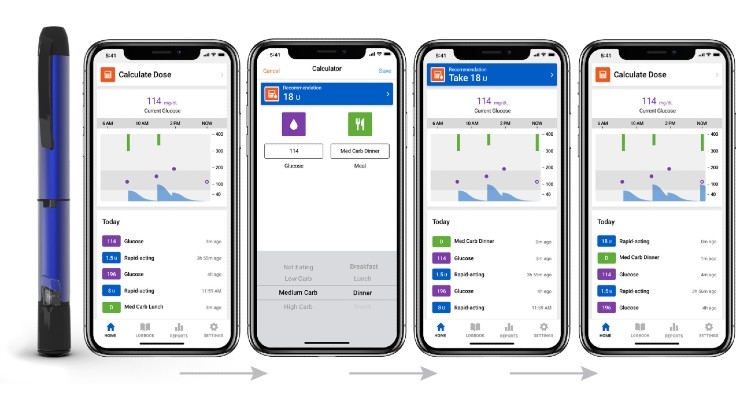
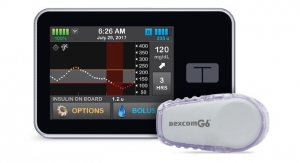
Companion Medical has received clearance from the FDA for its InPen bolus calculator for fixed dosing and meal estimation. The new bolus calculator takes into consideration each user's current glucose level and active insulin—the calculation of the amount of insulin still lowering glucose from previous bolus doses, removing the guesswork from insulin dosing. This is the first time a clearance like this has been issued for those with Type 1 or Type 2 insulin-dependent diabetes who administer a fixed amount of insulin for meals or deliver a dose based on the approximate size of their planned meal, as opposed to individual carb estimations. These new capabilities allow InPen to help the majority of patients using multiple daily injections, regardless of their personal insulin regimen.
"Our goal since launching in 2017 has been to make insulin therapy simpler for all people with diabetes—not just the most engaged or advanced. We do this by letting the InPen system do most of the heavy lifting and ease the burden," said Sean Saint, CEO and founder of Companion Medical. "Similar to the first InPen dose calculator, which has been a game-changer for those of us who count carbs, this new FDA clearance for users on fixed-dose or meal estimation therapy finally offers a simple, yet advanced solution to those people living with insulin-dependent diabetes who are not expert carb counters."
"The biggest predictor of better control amongst people living with diabetes is the number of insulin doses per day. Patients without a dose calculator with active insulin tracking have been taught for years to dose at least four hours apart because of the dangers of insulin stacking—this allows for only a maximum of three or four doses per day," said Mike Mensinger, CTO and co-founder at Companion Medical. "With the new calculator modes, InPen provides an experience consistent with these patients' current therapies, while adjusting recommendations automatically based on their current glucose level and factoring in active insulin to safely allow correction of high blood glucose between meals."
"This feature allows insulin pen users to be met where they are in their therapy," said Janice MacLeod who leads Companion Medical's clinical advocacy efforts. "The clinicians I have spoken to are excited about this feature for their patients, in particular those with Type 2 diabetes who inject insulin. Not all patients are able or wish to, count carbs for every meal, and the option of correction doses informed by InPen's active insulin tracking is incredibly useful in helping users avoid stacking their insulin and minimizing their risk of low glucose."
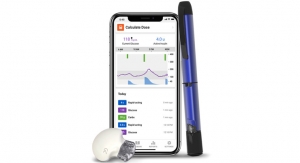
The InPen system, which includes a smart pen and Bluetooth connected app, helps calculate insulin doses, issue dose reminders, track active insulin and send reports to caregivers. InPen is available in the U.S. by prescription only. The app is available for download on the Apple App Store and Google Play.
Companion Medical has received clearance from the FDA for its InPen bolus calculator for fixed dosing and meal estimation. The new bolus calculator takes into consideration each user's current glucose level and active insulin—the calculation of the amount of insulin still lowering glucose from previous bolus doses, removing the guesswork from insulin dosing. This is the first time a clearance like this has been issued for those with Type 1 or Type 2 insulin-dependent diabetes who administer a fixed amount of insulin for meals or deliver a dose based on the approximate size of their planned meal, as opposed to individual carb estimations. These new capabilities allow InPen to help the majority of patients using multiple daily injections, regardless of their personal insulin regimen.
"Our goal since launching in 2017 has been to make insulin therapy simpler for all people with diabetes—not just the most engaged or advanced. We do this by letting the InPen system do most of the heavy lifting and ease the burden," said Sean Saint, CEO and founder of Companion Medical. "Similar to the first InPen dose calculator, which has been a game-changer for those of us who count carbs, this new FDA clearance for users on fixed-dose or meal estimation therapy finally offers a simple, yet advanced solution to those people living with insulin-dependent diabetes who are not expert carb counters."
"The biggest predictor of better control amongst people living with diabetes is the number of insulin doses per day. Patients without a dose calculator with active insulin tracking have been taught for years to dose at least four hours apart because of the dangers of insulin stacking—this allows for only a maximum of three or four doses per day," said Mike Mensinger, CTO and co-founder at Companion Medical. "With the new calculator modes, InPen provides an experience consistent with these patients' current therapies, while adjusting recommendations automatically based on their current glucose level and factoring in active insulin to safely allow correction of high blood glucose between meals."
"This feature allows insulin pen users to be met where they are in their therapy," said Janice MacLeod who leads Companion Medical's clinical advocacy efforts. "The clinicians I have spoken to are excited about this feature for their patients, in particular those with Type 2 diabetes who inject insulin. Not all patients are able or wish to, count carbs for every meal, and the option of correction doses informed by InPen's active insulin tracking is incredibly useful in helping users avoid stacking their insulin and minimizing their risk of low glucose."
The InPen system, which includes a smart pen and Bluetooth connected app, helps calculate insulin doses, issue dose reminders, track active insulin and send reports to caregivers. InPen is available in the U.S. by prescription only. The app is available for download on the Apple App Store and Google Play.