Business Wire10.31.19
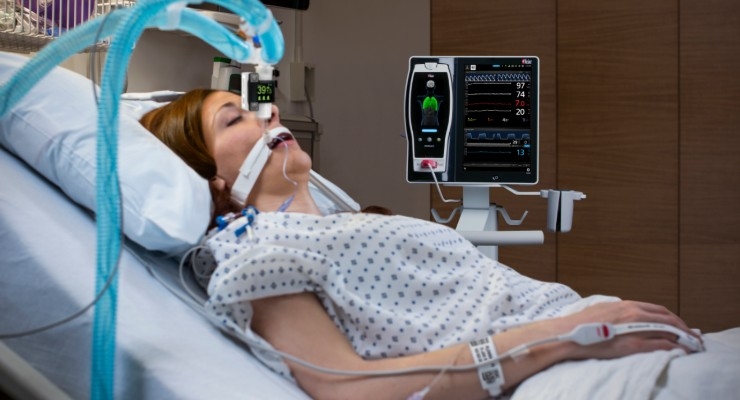
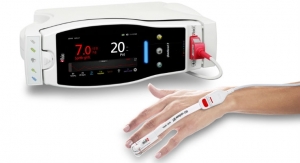
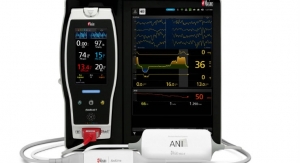
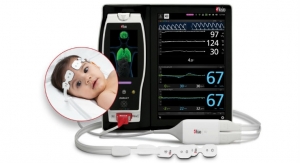
Masimo announced Radius Capnography, a portable real-time capnograph with wireless Bluetooth connectivity, has received CE marking. Radius Capnography connects with the Root Patient Monitoring and Connectivity Platform to provide seamless, tetherless mainstream capnography for patients of all ages. Radius Capnography is the second wireless sensor created by Masimo, joining Radius PPG, or Radius Photoplethysmography, the first tetherless sensor solution to offer Masimo SET Measure-through Motion and Low Perfusion pulse oximetry.
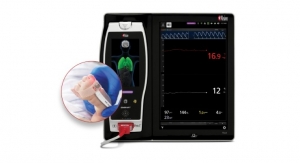
Radius Capnography requires no routine calibration and minimal warm-up time, with fully accurate EtCO2 and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds.1
Wirelessly connected to Root, Radius Capnography presents a compelling mainstream capnography solution:
American Heart Association guidelines recommend continuous quantitative waveform capnography, in addition to clinical assessment, to help clinicians confirm endotracheal tube placement3, assess the depth and effectiveness of chest compressions during CPR4, and recognize the return of spontaneous circulation during CPR.4
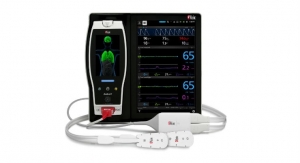
Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with Radius Capnography and other measurements, such as Radius PPG SET Measure-through Motion and Low Perfusion pulse oximetry and advanced rainbow Pulse CO-Oximetry measurements, for expanded visibility of patient status.
Joe Kiani, founder and CEO of Masimo, said, “We’re happy to introduce the second tetherless, cableless sensor for Root, Radius Capnography. With this CE marking, the advanced connectivity of Radius Capnography is now available in both the US and CE-marked countries, bringing the power of Masimo capnography to even more hospitals around the world.”
References
1 EMMA Ooperator’s Manual.
2 The Value of Medical Device Interoperability. West Health Institute. 1013.
3 Link MS, et al. Circulation. 2015; 132(suppl 2): S444–S464.
4 Neumar RW et al. Circulation. 2010;122:S729-S767.
Radius Capnography requires no routine calibration and minimal warm-up time, with fully accurate EtCO2 and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds.1
Wirelessly connected to Root, Radius Capnography presents a compelling mainstream capnography solution:
- Cable-free Capnography: The lack of a cable tethering the Radius Capnography sensor and patient to Root improves workflow and reduces the possibility of an interruption in capnography monitoring by minimizing tugging on the breathing circuit.
- Automated Documentation: Root automates electronic charting of patient data, including the data collected by Radius Capnography, in hospital electronic medical record (EMR) systems, working with Masimo Patient SafetyNet or Iris Gateway to simplify and speed workflow, as well as reduce the likelihood of transcription errors.2
- Maximized Data Visibility and Manipulation: Root’s large, multi-touch high-resolution screen provides an easily interpretable secondary display of large, crisp EtCO2 waveforms, improving visibility and assisting clinicians in identifying wave patterns suggestive of airway obstruction or tube dislodgement. Clearly displayed trend data for up to 96 hours helps clinicians review patient progress over time, helping guide ventilation efforts. And the intuitive touch-screen interface allows clinicians to quickly adjust the trend display range and configure alarm settings to meet the needs of each patient.
- Hassle-free Connectivity: Radius Capnography’s cable-free design and quick Bluetooth pairing provide the benefits of reliable capnography and connection to Root without the burden or clutter of additional cables, facilitating easy movement between care areas, as patients move through the hospital, and in busy operating rooms where space is already at a premium.
American Heart Association guidelines recommend continuous quantitative waveform capnography, in addition to clinical assessment, to help clinicians confirm endotracheal tube placement3, assess the depth and effectiveness of chest compressions during CPR4, and recognize the return of spontaneous circulation during CPR.4
Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with Radius Capnography and other measurements, such as Radius PPG SET Measure-through Motion and Low Perfusion pulse oximetry and advanced rainbow Pulse CO-Oximetry measurements, for expanded visibility of patient status.
Joe Kiani, founder and CEO of Masimo, said, “We’re happy to introduce the second tetherless, cableless sensor for Root, Radius Capnography. With this CE marking, the advanced connectivity of Radius Capnography is now available in both the US and CE-marked countries, bringing the power of Masimo capnography to even more hospitals around the world.”
References
1 EMMA Ooperator’s Manual.
2 The Value of Medical Device Interoperability. West Health Institute. 1013.
3 Link MS, et al. Circulation. 2015; 132(suppl 2): S444–S464.
4 Neumar RW et al. Circulation. 2010;122:S729-S767.