Business Wire10.22.19
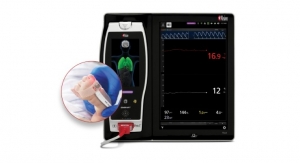
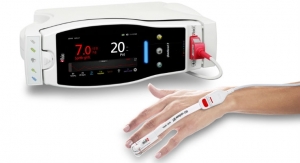
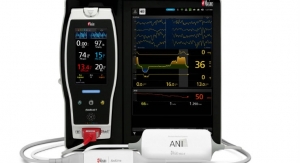
Masimo has announced three additional indices (delta cHb, delta HHb, and delta O2Hb) for O3 Regional Oximetry. These indices provide clinicians with additional visibility into changes in the underlying oxyhemoglobin and deoxyhemoglobin components used to calculate cerebral oxygen saturation, rSO2. With these additions, clinicians will now be able to view the relative contribution of each component to a patient’s overall rSO2. O3, available on the Masimo Root Patient Monitoring and Connectivity Platform, is cleared by the U.S. Food and Drug Administration (FDA) for the monitoring of cerebral oxygenation and may be helpful in situations in which peripheral pulse oximetry alone may not be fully indicative of the oxygenation of the brain.
O3 uses near-infrared spectroscopy (NIRS) to monitor and display continuous rSO2 values for each side of the brain. As the degree of oxygenation in cerebral tissue changes, the wavelengths of light absorbed by that tissue and those returned to the O3 sensors also change, forming the basis for the measurement of regional (cerebral) oxygen saturation, rSO2. Until now, rSO2 has been displayed as a single, continuous value for each side of the brain. With these three new indices, O3 can now display information about the changes in the underlying components used to calculate rSO2 values. Delta O2Hb provides an index representing changes in the oxyhemoglobin component of the rSO2 calculation. Delta HHb provides an index representing changes in the deoxyhemoglobin component of the rSO2 calculation. Finally, delta cHb provides an index representing the sum of delta O2Hb and delta HHb.
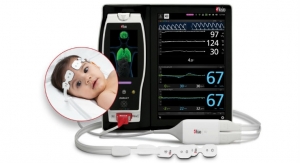
O3 is available as a Masimo Open Connect (MOC-9) module for Root, a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3 and other measurements, such as SedLine brain function monitoring— for a more complete picture of the brain—and SET Measure-through Motion and Low Perfusion pulse oximetry, for expanded visibility of oxygenation status. O3 is available for all patient populations, with sensors in three sizes, for adult (≥40 kg), pediatric (≥5 kg and <40 kg), and infant and neonatal (<10 kg) patients.
Joe Kiani, Founder and CEO of Masimo, said, “We are proud to announce these three O3 indices, which we developed in response to requests from clinicians. Now, for the first time, clinicians can monitor not just overall cerebral oxygen saturation but also have access to additional data on the changes in the underlying oxyhemoglobin and deoxyhemoglobin components that make up rSO2 values—data that we hope can help provide additional insight into patient status.”
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces monitoring technologies, including measurements, sensors, patient monitors, and automation and connectivity solutions. Its mission is to improve patient outcomes and reduce the cost of care. Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET is estimated to be used on more than 100 million patients in hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at nine of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET and in 2018, announced that SpO2 accuracy on RD SET sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), RPVi (rainbow PVi), and Oxygen Reserve Index (ORi). In 2013, Masimo introduced the Root Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine Brain Function Monitoring, O3 Regional Oximetry, and ISA Capnography with NomoLine sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7 and Radius PPG, portable devices like Rad-67, fingertip pulse oximeters like MightySat Rx, and devices available for use both in the hospital and at home, such as Rad-97. Masimo hospital automation and connectivity solutions are centered around the Iris platform, and include Iris Gateway, Patient SafetyNet, Replica, Halo ION, UniView, and Doctella.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1 Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2 Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3 de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4 Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5 Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6 McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7 Estimate: Masimo data on file.
8 http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
O3 uses near-infrared spectroscopy (NIRS) to monitor and display continuous rSO2 values for each side of the brain. As the degree of oxygenation in cerebral tissue changes, the wavelengths of light absorbed by that tissue and those returned to the O3 sensors also change, forming the basis for the measurement of regional (cerebral) oxygen saturation, rSO2. Until now, rSO2 has been displayed as a single, continuous value for each side of the brain. With these three new indices, O3 can now display information about the changes in the underlying components used to calculate rSO2 values. Delta O2Hb provides an index representing changes in the oxyhemoglobin component of the rSO2 calculation. Delta HHb provides an index representing changes in the deoxyhemoglobin component of the rSO2 calculation. Finally, delta cHb provides an index representing the sum of delta O2Hb and delta HHb.
O3 is available as a Masimo Open Connect (MOC-9) module for Root, a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3 and other measurements, such as SedLine brain function monitoring— for a more complete picture of the brain—and SET Measure-through Motion and Low Perfusion pulse oximetry, for expanded visibility of oxygenation status. O3 is available for all patient populations, with sensors in three sizes, for adult (≥40 kg), pediatric (≥5 kg and <40 kg), and infant and neonatal (<10 kg) patients.
Joe Kiani, Founder and CEO of Masimo, said, “We are proud to announce these three O3 indices, which we developed in response to requests from clinicians. Now, for the first time, clinicians can monitor not just overall cerebral oxygen saturation but also have access to additional data on the changes in the underlying oxyhemoglobin and deoxyhemoglobin components that make up rSO2 values—data that we hope can help provide additional insight into patient status.”
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces monitoring technologies, including measurements, sensors, patient monitors, and automation and connectivity solutions. Its mission is to improve patient outcomes and reduce the cost of care. Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET is estimated to be used on more than 100 million patients in hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at nine of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET and in 2018, announced that SpO2 accuracy on RD SET sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), RPVi (rainbow PVi), and Oxygen Reserve Index (ORi). In 2013, Masimo introduced the Root Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine Brain Function Monitoring, O3 Regional Oximetry, and ISA Capnography with NomoLine sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7 and Radius PPG, portable devices like Rad-67, fingertip pulse oximeters like MightySat Rx, and devices available for use both in the hospital and at home, such as Rad-97. Masimo hospital automation and connectivity solutions are centered around the Iris platform, and include Iris Gateway, Patient SafetyNet, Replica, Halo ION, UniView, and Doctella.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1 Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2 Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3 de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4 Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5 Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6 McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7 Estimate: Masimo data on file.
8 http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.