Michael Barbella, Managing Editor03.24.21
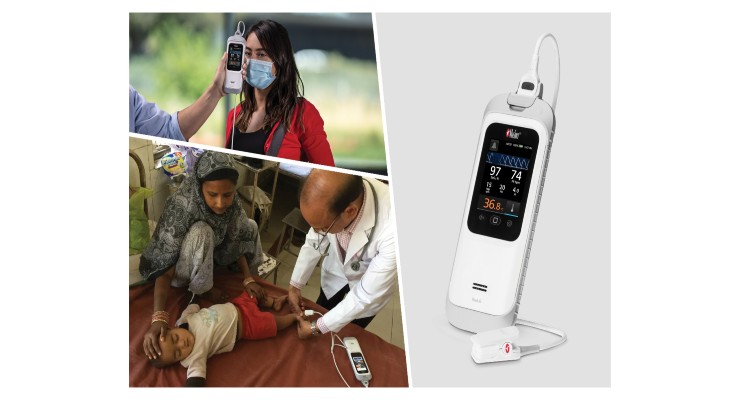
European regulators have given their approval to Masimo's handheld Rad-G with Temperature device.
The company recently recented CE Marking for the product, which provides clinically proven SET pulse oximetry, respiration rate from the pleth (RRp), and other important parameters alongside clinical-grade, non-contact infrared thermometry. A long-lasting rechargeable battery, rubber casing, and integrated noninvasive, real-time forehead temperature measurement, the Rad-G with Temperature makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor. Coupled with the universal Mini-Clip pulse oximeter sensor to provide the ultimate in handheld versatility, Rad-G with Temperature can be used in a variety of settings, including but not limited to entry screening, physicians’ offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments both indoors and in the field. Rad-G can provide both spot-check measurement and continuous monitoring.
“In the places where I’ve worked around the world, there has always been a demand for tools that enable the continuous monitoring of key vital signs, like respiration rate, oxygen saturation, and temperature, which can help providers and patients fight against illnesses from pneumonia to congenital heart disease,” said Paul Farmer, Kolokotrones University professor at Harvard, chair of the Department of Global Health and Social Medicine at Harvard Medical School, chief of the Division of Global Health Equity at Brigham and Women’s Hospital in Boston, and co-founder and chief strategist of Partners in Health.
Infrared thermometry offered by Rad-G with Temperature provides a host of benefits. Rad-G’s thermometer is non-contact and does not require probe covers or other disposable accessories. Its integration into the Rad-G platform eliminates the need for clinicians to locate a separate clinical thermometer to take body temperature measurements and ensures that many people can be seamlessly and efficiently screened for temperature, with one-touch operation, alongside oxygen saturation, respiration rate, and more, in the same session, using a single device. Designed from the start to maximize portability and battery life, Rad-G’s rechargeable battery provides 24 hours of continuous use between charges – allowing clinicians to work in transport, emergency, and other challenging scenarios with confidence that the device will continue to function hour after hour.
Joe Kiani, founder and CEO of Masimo, said, “With Rad-G, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings to serve the 5 billion people on our planet that to date have not had access to pulse oximetry, let alone SET pulse oximetry. With the addition of temperature measurement, Rad-G is more versatile than ever, streamlining the assessment of multiple key vital signs. Many caregivers travel miles, sometimes on bike, sometimes on foot, to help patients, so having a product that is light, small, multifunctional, and ‘accurate when you need it most’ is crucial. Rad-G was designed to be just that.”
First developed in partnership with The Bill & Melinda Gates Foundation as a spot-check device for use in pneumonia screening, the Rad-G with Temperature expands on its predecessor’s capabilities not only with the ability to measure temperature, but the addition of alarms, and thus the ability to provide both continuous monitoring and spot-check measurement – without sacrificing any portability, convenience, or ruggedness. Using the included power adapter, Rad-G can be converted from a handheld, spot-check device into a continuous monitoring device, in the absence of other multi-parameter monitors. As of 2010 – 20 years after use of pulse oximetry during surgery became routine in affluent countries – more than 77,000 operating theaters in low- and middle-income countries were still conducting surgery without pulse oximetry.1 Working with a myriad of non-profit organizations, Rad-G is being made available at an affordable price so the 5 billion people who don’t have access to reliable pulse oximetry can finally have it. When used for continuous monitoring, the high-resolution screen displays a continuous pleth waveform and the fully configurable, audible alarms help alert clinicians to changes in patient status that may require their intervention.
The development of Rad-G stems in part from the findings of a multi-center, prospective, two-stage observation study funded by The Bill & Melinda Gates Foundation, whose protocols were published in JMIR Research Protocols, in which Dr. Kevin Baker, MA, MSc, senior research specialist at the Malaria Consortium, and colleagues sought to identify the most accurate, usable, and acceptable devices to aid community health workers in the diagnosis of pneumonia symptoms in resource-poor settings.2 The researchers found that “The Masimo mobile phone pulse oximeter [iSpO2 Rx] had the best overall performance across all measures and in both age strata of the children the device was tested on. This may be due to the motion signal processing techniques incorporated in Masimo pulse oximeters which attempts to reduce motion artefact, which may be particularly important when using these devices on moving children.”3
Eric D. McCollum, M.D., MPH, director of the Global Program in Respiratory Sciences at the Johns Hopkins School of Medicine in Baltimore, Maryland, said, “The Masimo Rad-G is a fantastic device that is thoughtfully crafted and user-friendly for both healthcare workers with diverse training backgrounds and pediatric patients across the age spectrum. We are using the Rad-G currently in four countries in our pediatric global health work and the device is no doubt at the high standards set by Masimo with its range of high-quality pulse oximeters. The healthcare providers and children love it.”
SpO2 and PR monitoring on Rad-G is provided using clinically proven Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other technologies.4 SET is estimated to be used on more than 200 million patients a year5 and is the primary pulse oximetry at nine of the 10 hospitals that top the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.6 With Masimo SET technology in Rad-G, clinicians have access to accurate pulse oximetry measurements in the palm of the hand.
“Bacterial and viral pneumonias – including those caused by COVID-19 – are a leading cause of death in children and adults globally, with a disproportionate burden of disease in low-resource settings,” said Peter Moschovis, M.D., MPH, a pulmonologist at Massachusetts General Hospital. “Pulse oximetry plays an important role in the triage and management of patients with pneumonia.”
In a new cross-sectional study published in Acta Paediatrica, Dr. Baker and colleagues assessed the utility of Rad-G by observing how it was used by healthcare workers screening children under five for pneumonia in three regions of Ethiopia in 2018.7 The researchers found that healthcare workers gave correct treatment and referral guidance using Rad-G’s results and their assessment of other symptoms in 94.9 percent and 95.8 percent of cases in the first and second of their two observation groups, respectively.
In addition to temperature measurements and Masimo SET oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and PVi (for assessing fluid responsiveness), the same SpO2 sensor can be used to monitor respiration rate from the plethysmograph, with RRp. Difficulty breathing and fever are generally considered two of the earliest signs of patient deterioration, and Masimo hopes that the availability of RRp and thermometry on Rad-G may play a role in assisting clinicians and public health officials as they seek to combat numerous types of illnesses, including pneumonia and COVID-19.
Rad-G with Temperature can be used with a variety of reusable and single-patient use sensors. The universal direct-connect Rad-G reusable sensor, indicated for monitoring adult, pediatric, and infant patients, helps to eliminate the need to stock and carry multiple sensor types, increasing the device’s versatility and ease of use, especially in more challenging field environments. Rad-G with Temperature is also compatible with the vast portfolio of Masimo single-patient-use adhesive sensors—including Masimo RD SET sensors, which offer best-in-class accuracy specifications of 1.5 percent in conditions of motion and no motion—ensuring clinicians can customize their setup based on the unique needs of each care setting. In addition, Rad-G is designed to work reliably on all people, from white to black, neonate to geriatric.
Rad-G is U.S. Food and Drug Administration (FDA) 510(k) cleared and is available in the United States. Rad-G with Temperature has not received FDA 510(k) clearance and is not currently available in the United States. PVi is FDA 510(k) cleared as an indicator of fluid responsiveness in select populations of mechanically ventilated adult patients in the United States.
References
1 https://www.theatlantic.com/health/archive/2017/02/pulse-oximeter/516510/
2 Baker K, Akasiima M, Wharton-Smith A, Habte T, Matata L, Nanyumba N, Okwir M, Sebsibe A, Marasciulo M, Petzold M, Källander K. “Performance, Acceptability, and Usability of Respiratory Rate Timers and Pulse Oximeters When Used by Frontline Health Workers to Detect Symptoms of Pneumonia in Sub-Saharan Africa and Southeast Asia: Protocol for a Two-Phase Multisite, Mixed-Methods Trial.” JMIR Res Protoc. 2018;7(10):e10191) doi: 10.2196/10191.
3 https://openarchive.ki.se/xmlui/bitstream/handle/10616/46833/Thesis_Kevin_Baker.pdf?sequence=4&isAllowed=y
4 Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5 Estimate: Masimo data on file.
6 http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
7 Baker K, Ward C, Maurel A, de Cola M, Smith H, Getachew D, Habte T, McWhorter C, LaBarre P, Karlstrom J, Ameha A, Tariku A, Black J, Bassat Q, Kallander K. “Usability and acceptability of a multimodal respiratory rate and pulse oximeter device in case management of children with symptoms of pneumonia: A cross-sectional study in Ethiopia.” Acta Paediatrica. 19 Nov 2020. DOI: 10.1111/apa.15682
The company recently recented CE Marking for the product, which provides clinically proven SET pulse oximetry, respiration rate from the pleth (RRp), and other important parameters alongside clinical-grade, non-contact infrared thermometry. A long-lasting rechargeable battery, rubber casing, and integrated noninvasive, real-time forehead temperature measurement, the Rad-G with Temperature makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor. Coupled with the universal Mini-Clip pulse oximeter sensor to provide the ultimate in handheld versatility, Rad-G with Temperature can be used in a variety of settings, including but not limited to entry screening, physicians’ offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments both indoors and in the field. Rad-G can provide both spot-check measurement and continuous monitoring.
“In the places where I’ve worked around the world, there has always been a demand for tools that enable the continuous monitoring of key vital signs, like respiration rate, oxygen saturation, and temperature, which can help providers and patients fight against illnesses from pneumonia to congenital heart disease,” said Paul Farmer, Kolokotrones University professor at Harvard, chair of the Department of Global Health and Social Medicine at Harvard Medical School, chief of the Division of Global Health Equity at Brigham and Women’s Hospital in Boston, and co-founder and chief strategist of Partners in Health.
Infrared thermometry offered by Rad-G with Temperature provides a host of benefits. Rad-G’s thermometer is non-contact and does not require probe covers or other disposable accessories. Its integration into the Rad-G platform eliminates the need for clinicians to locate a separate clinical thermometer to take body temperature measurements and ensures that many people can be seamlessly and efficiently screened for temperature, with one-touch operation, alongside oxygen saturation, respiration rate, and more, in the same session, using a single device. Designed from the start to maximize portability and battery life, Rad-G’s rechargeable battery provides 24 hours of continuous use between charges – allowing clinicians to work in transport, emergency, and other challenging scenarios with confidence that the device will continue to function hour after hour.
Joe Kiani, founder and CEO of Masimo, said, “With Rad-G, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings to serve the 5 billion people on our planet that to date have not had access to pulse oximetry, let alone SET pulse oximetry. With the addition of temperature measurement, Rad-G is more versatile than ever, streamlining the assessment of multiple key vital signs. Many caregivers travel miles, sometimes on bike, sometimes on foot, to help patients, so having a product that is light, small, multifunctional, and ‘accurate when you need it most’ is crucial. Rad-G was designed to be just that.”
First developed in partnership with The Bill & Melinda Gates Foundation as a spot-check device for use in pneumonia screening, the Rad-G with Temperature expands on its predecessor’s capabilities not only with the ability to measure temperature, but the addition of alarms, and thus the ability to provide both continuous monitoring and spot-check measurement – without sacrificing any portability, convenience, or ruggedness. Using the included power adapter, Rad-G can be converted from a handheld, spot-check device into a continuous monitoring device, in the absence of other multi-parameter monitors. As of 2010 – 20 years after use of pulse oximetry during surgery became routine in affluent countries – more than 77,000 operating theaters in low- and middle-income countries were still conducting surgery without pulse oximetry.1 Working with a myriad of non-profit organizations, Rad-G is being made available at an affordable price so the 5 billion people who don’t have access to reliable pulse oximetry can finally have it. When used for continuous monitoring, the high-resolution screen displays a continuous pleth waveform and the fully configurable, audible alarms help alert clinicians to changes in patient status that may require their intervention.
The development of Rad-G stems in part from the findings of a multi-center, prospective, two-stage observation study funded by The Bill & Melinda Gates Foundation, whose protocols were published in JMIR Research Protocols, in which Dr. Kevin Baker, MA, MSc, senior research specialist at the Malaria Consortium, and colleagues sought to identify the most accurate, usable, and acceptable devices to aid community health workers in the diagnosis of pneumonia symptoms in resource-poor settings.2 The researchers found that “The Masimo mobile phone pulse oximeter [iSpO2 Rx] had the best overall performance across all measures and in both age strata of the children the device was tested on. This may be due to the motion signal processing techniques incorporated in Masimo pulse oximeters which attempts to reduce motion artefact, which may be particularly important when using these devices on moving children.”3
Eric D. McCollum, M.D., MPH, director of the Global Program in Respiratory Sciences at the Johns Hopkins School of Medicine in Baltimore, Maryland, said, “The Masimo Rad-G is a fantastic device that is thoughtfully crafted and user-friendly for both healthcare workers with diverse training backgrounds and pediatric patients across the age spectrum. We are using the Rad-G currently in four countries in our pediatric global health work and the device is no doubt at the high standards set by Masimo with its range of high-quality pulse oximeters. The healthcare providers and children love it.”
SpO2 and PR monitoring on Rad-G is provided using clinically proven Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other technologies.4 SET is estimated to be used on more than 200 million patients a year5 and is the primary pulse oximetry at nine of the 10 hospitals that top the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.6 With Masimo SET technology in Rad-G, clinicians have access to accurate pulse oximetry measurements in the palm of the hand.
“Bacterial and viral pneumonias – including those caused by COVID-19 – are a leading cause of death in children and adults globally, with a disproportionate burden of disease in low-resource settings,” said Peter Moschovis, M.D., MPH, a pulmonologist at Massachusetts General Hospital. “Pulse oximetry plays an important role in the triage and management of patients with pneumonia.”
In a new cross-sectional study published in Acta Paediatrica, Dr. Baker and colleagues assessed the utility of Rad-G by observing how it was used by healthcare workers screening children under five for pneumonia in three regions of Ethiopia in 2018.7 The researchers found that healthcare workers gave correct treatment and referral guidance using Rad-G’s results and their assessment of other symptoms in 94.9 percent and 95.8 percent of cases in the first and second of their two observation groups, respectively.
In addition to temperature measurements and Masimo SET oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and PVi (for assessing fluid responsiveness), the same SpO2 sensor can be used to monitor respiration rate from the plethysmograph, with RRp. Difficulty breathing and fever are generally considered two of the earliest signs of patient deterioration, and Masimo hopes that the availability of RRp and thermometry on Rad-G may play a role in assisting clinicians and public health officials as they seek to combat numerous types of illnesses, including pneumonia and COVID-19.
Rad-G with Temperature can be used with a variety of reusable and single-patient use sensors. The universal direct-connect Rad-G reusable sensor, indicated for monitoring adult, pediatric, and infant patients, helps to eliminate the need to stock and carry multiple sensor types, increasing the device’s versatility and ease of use, especially in more challenging field environments. Rad-G with Temperature is also compatible with the vast portfolio of Masimo single-patient-use adhesive sensors—including Masimo RD SET sensors, which offer best-in-class accuracy specifications of 1.5 percent in conditions of motion and no motion—ensuring clinicians can customize their setup based on the unique needs of each care setting. In addition, Rad-G is designed to work reliably on all people, from white to black, neonate to geriatric.
Rad-G is U.S. Food and Drug Administration (FDA) 510(k) cleared and is available in the United States. Rad-G with Temperature has not received FDA 510(k) clearance and is not currently available in the United States. PVi is FDA 510(k) cleared as an indicator of fluid responsiveness in select populations of mechanically ventilated adult patients in the United States.
References
1 https://www.theatlantic.com/health/archive/2017/02/pulse-oximeter/516510/
2 Baker K, Akasiima M, Wharton-Smith A, Habte T, Matata L, Nanyumba N, Okwir M, Sebsibe A, Marasciulo M, Petzold M, Källander K. “Performance, Acceptability, and Usability of Respiratory Rate Timers and Pulse Oximeters When Used by Frontline Health Workers to Detect Symptoms of Pneumonia in Sub-Saharan Africa and Southeast Asia: Protocol for a Two-Phase Multisite, Mixed-Methods Trial.” JMIR Res Protoc. 2018;7(10):e10191) doi: 10.2196/10191.
3 https://openarchive.ki.se/xmlui/bitstream/handle/10616/46833/Thesis_Kevin_Baker.pdf?sequence=4&isAllowed=y
4 Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5 Estimate: Masimo data on file.
6 http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
7 Baker K, Ward C, Maurel A, de Cola M, Smith H, Getachew D, Habte T, McWhorter C, LaBarre P, Karlstrom J, Ameha A, Tariku A, Black J, Bassat Q, Kallander K. “Usability and acceptability of a multimodal respiratory rate and pulse oximeter device in case management of children with symptoms of pneumonia: A cross-sectional study in Ethiopia.” Acta Paediatrica. 19 Nov 2020. DOI: 10.1111/apa.15682