Business Wire10.08.19
Dexcom Inc. announced the U.S. Food and Drug Administration (FDA) has cleared the Dexcom G6 Pro Continuous Glucose Monitoring (CGM) System for healthcare professionals to use with their patients, ages two years and up.
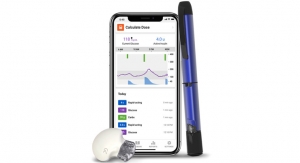
Dexcom G6 Pro is the first and only single use, professional CGM that gathers real-time glucose data over a 10-day period and offers both a blinded and unblinded mode. In blinded mode, real-time glucose data is hidden from the patient and reviewed retrospectively with their healthcare professional at the end of the session. In unblinded mode, patients can see their glucose data throughout the 10-day sensor wear to gain insights and make treatment decisions in real time.
Regardless of mode, glucose data gathered by the Dexcom G6 Pro enables providers to adjust a patient’s diabetes therapy plan with precision and customization. Providers can also use the data to help patients modify their daily behavior after seeing the effects that variables such as food, exercise, stress and medication have on glucose levels.
“Dexcom G6 Pro will enable healthcare providers to set up their patients with CGM in minutes,” said Davida Kruger, MSN, APN-BC, BC-ADM, diabetes specialist, Division of Endocrinology, Diabetes, Bone and Mineral Disorders at Henry Ford Health System in Detroit. “This new professional system will serve as a simple way to obtain data from CGM naïve patients who need glucose insight, but don’t need or want to be monitored around the clock. It will give all my patients a chance to try the Dexcom G6 Pro under a healthcare provider’s supervision before they commit to a personal system.”
Dexcom G6 Pro features and benefits:
“At Dexcom, we are continuing to drive innovation in wearable health technology,” said Kevin Sayer, president, CEO and chairman of Dexcom. “Using the power of Dexcom G6 Pro, clinicians can use the insights gained from a 10-day professional CGM session to adjust treatment plans and empower their patients to live healthier lives.”
The company expects to begin shipping Dexcom G6 Pro early next year.
*If your glucose alerts and readings from the Dexcom G6 do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
† For a list of compatible devices, visit www.dexcom.com/compatibility.
Dexcom G6 Pro is the first and only single use, professional CGM that gathers real-time glucose data over a 10-day period and offers both a blinded and unblinded mode. In blinded mode, real-time glucose data is hidden from the patient and reviewed retrospectively with their healthcare professional at the end of the session. In unblinded mode, patients can see their glucose data throughout the 10-day sensor wear to gain insights and make treatment decisions in real time.
Regardless of mode, glucose data gathered by the Dexcom G6 Pro enables providers to adjust a patient’s diabetes therapy plan with precision and customization. Providers can also use the data to help patients modify their daily behavior after seeing the effects that variables such as food, exercise, stress and medication have on glucose levels.
“Dexcom G6 Pro will enable healthcare providers to set up their patients with CGM in minutes,” said Davida Kruger, MSN, APN-BC, BC-ADM, diabetes specialist, Division of Endocrinology, Diabetes, Bone and Mineral Disorders at Henry Ford Health System in Detroit. “This new professional system will serve as a simple way to obtain data from CGM naïve patients who need glucose insight, but don’t need or want to be monitored around the clock. It will give all my patients a chance to try the Dexcom G6 Pro under a healthcare provider’s supervision before they commit to a personal system.”
Dexcom G6 Pro features and benefits:
- Easy sensor applicator – Allows for one-touch, simple insertion
- Single-use, disposable sensor – Simplifies session start and end for both patients and providers
- Auto-start transmitter – Expedites startup time to get patients on CGM quickly and easily
- Blinded or unblinded mode – Blinded: patients wear a sensor for 10 days, then return to their physician’s office to review the data retrospectively (indicated for assessing glycemic variability in persons age two years and up). Unblinded: patients can view glucose data in real-time on a simplified mobile app* throughout the 10-day sensor wear (indicated for management of diabetes, in persons age two years and up)
- Continuous glucose readings – Reads glucose values automatically every five minutes
- Alerts and alarms – Warns user of dangerous high and low glucose levels (unblinded mode only)
- Fingerstick elimination – No fingersticks needed for calibration or diabetes treatment decisions†
- Dexcom CLARITY – Allows providers to pull interactive reports and review glucose patterns, trends and statistics retrospectively with their patients
“At Dexcom, we are continuing to drive innovation in wearable health technology,” said Kevin Sayer, president, CEO and chairman of Dexcom. “Using the power of Dexcom G6 Pro, clinicians can use the insights gained from a 10-day professional CGM session to adjust treatment plans and empower their patients to live healthier lives.”
The company expects to begin shipping Dexcom G6 Pro early next year.
*If your glucose alerts and readings from the Dexcom G6 do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
† For a list of compatible devices, visit www.dexcom.com/compatibility.