Business Wire09.26.19
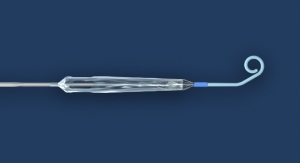
Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for safety and efficacy in the therapy of cardiogenic shock for up to 14 days.
Impella 5.5 with SmartAssist is:
“A minimally invasive, forward flow, fully unloading heart pump that is designed for surgery is game changing,” said Mark Anderson, M.D., chief of the Division of Cardiac Surgery and cardiothoracic surgeon at the Heart and Vascular Hospital at Hackensack University Medical Center and Hackensack Meridian Health. “This gives cardiac surgeons a new and potentially better option that can provide the benefits of heart recovery to some of our sickest patients.”
“The Impella 5.5 is designed to give severely ill patients the best chance for recovery of the heart," said Hermann Reichenspurner, MD, PhD, professor and chief, Department of Cardiovascular Surgery, University Heart Center Hamburg. "A forward flow, minimally invasive heart pump that unloads the left ventricle and perfuses the end organs adequately is an ideal tool to help stabilize a patient after cardiac decompensation and give the heart time to rest and recover."
The FDA indication for use of Impella 5.5 with SmartAssist is as follows:
The Impella 5.5 with SmartAssist System is a temporary ventricular support device intended for short term (14 days) use and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery or in the setting of cardiomyopathy, including peripartum cardiomyopathy, or myocarditis as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures (including volume loading and use of pressors and inotropes, with or without IABP). The intent of Impella System Therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
Impella 5.5 with SmartAssist delivers peak flows of greater than 6 liters per minute. A motor housing that is thinner and 45 percent shorter than the Impella 5.0 improves ease of pump insertion through the vasculature.
The inclusion of SmartAssist enables intelligent decision making with weaning algorithms designed to increase survival with heart recovery. SmartAssist also integrates data informatics including left ventricular pressure (LVP), end-diastolic pressure (EDP) and cardiac power output (CPO). The SmartAssist fiberoptic pressure sensor allows for precise pump positioning, management and repositioning in the ICU. Impella Connect enables clinicians to view the Impella control screen through a secure, HIPAA compliant website to track and review cases at any time from any internet-connected device.
The Impella 5.5 with SmartAssist will be introduced in the United States through a controlled rollout at hospitals with established heart recovery protocols. Impella 5.5 with SmartAssist received CE marking approval in Europe in April 2018 and was introduced in Germany through a similar controlled rollout.
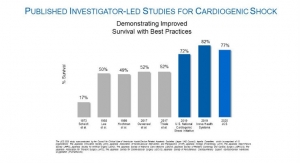
The supplement approval for the Impella 5.5 with SmartAssist stems from the FDA’s Impella PMA approvals indicating Impella devices as safe and effective for the treatment of cardiogenic shock. The approvals were based on analyses of Impella clinical results in 508 patients, which includes the FDA study RECOVER I, and the U.S. Impella registry, and of Impella literature reviews of 801 patients in 33 clinical studies. Additionally, more than 49,000 patients supported by Impella technology were reviewed in a safety analysis.
Impella 5.5 with SmartAssist is:
- Minimally invasive, eliminating the need for a sternotomy or coring of the left ventricle
- Designed for heart surgeons, implanted via the axillary artery or the anterior aorta
- Forward flow, to provide the patient with coronary flow and renal perfusion
- Fully unloading, to reduce the heart’s oxygen demand and work
- Equipped with SmartAssist, designed to provide weaning algorithms to optimize survival and native heart recovery
“A minimally invasive, forward flow, fully unloading heart pump that is designed for surgery is game changing,” said Mark Anderson, M.D., chief of the Division of Cardiac Surgery and cardiothoracic surgeon at the Heart and Vascular Hospital at Hackensack University Medical Center and Hackensack Meridian Health. “This gives cardiac surgeons a new and potentially better option that can provide the benefits of heart recovery to some of our sickest patients.”
“The Impella 5.5 is designed to give severely ill patients the best chance for recovery of the heart," said Hermann Reichenspurner, MD, PhD, professor and chief, Department of Cardiovascular Surgery, University Heart Center Hamburg. "A forward flow, minimally invasive heart pump that unloads the left ventricle and perfuses the end organs adequately is an ideal tool to help stabilize a patient after cardiac decompensation and give the heart time to rest and recover."
The FDA indication for use of Impella 5.5 with SmartAssist is as follows:
The Impella 5.5 with SmartAssist System is a temporary ventricular support device intended for short term (14 days) use and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery or in the setting of cardiomyopathy, including peripartum cardiomyopathy, or myocarditis as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures (including volume loading and use of pressors and inotropes, with or without IABP). The intent of Impella System Therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
Impella 5.5 with SmartAssist delivers peak flows of greater than 6 liters per minute. A motor housing that is thinner and 45 percent shorter than the Impella 5.0 improves ease of pump insertion through the vasculature.
The inclusion of SmartAssist enables intelligent decision making with weaning algorithms designed to increase survival with heart recovery. SmartAssist also integrates data informatics including left ventricular pressure (LVP), end-diastolic pressure (EDP) and cardiac power output (CPO). The SmartAssist fiberoptic pressure sensor allows for precise pump positioning, management and repositioning in the ICU. Impella Connect enables clinicians to view the Impella control screen through a secure, HIPAA compliant website to track and review cases at any time from any internet-connected device.
The Impella 5.5 with SmartAssist will be introduced in the United States through a controlled rollout at hospitals with established heart recovery protocols. Impella 5.5 with SmartAssist received CE marking approval in Europe in April 2018 and was introduced in Germany through a similar controlled rollout.
The supplement approval for the Impella 5.5 with SmartAssist stems from the FDA’s Impella PMA approvals indicating Impella devices as safe and effective for the treatment of cardiogenic shock. The approvals were based on analyses of Impella clinical results in 508 patients, which includes the FDA study RECOVER I, and the U.S. Impella registry, and of Impella literature reviews of 801 patients in 33 clinical studies. Additionally, more than 49,000 patients supported by Impella technology were reviewed in a safety analysis.