Business Wire07.01.20
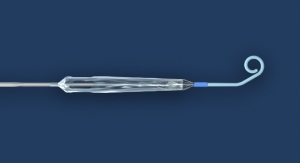
Abiomed, maker of the Impella heart pump, has acquired Breethe, developer of an extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation, including patients suffering from cardiogenic shock or respiratory failure such as due to ARDS, H1N1, SARS, or COVID-19. ECMO has also been utilized as a primary method of oxygenation and hemodynamic support for pediatric patients.
Patients who require ECMO therapy have a severe and life-threatening illness that stops their lungs from working properly. The system is connected to patients through tubes (cannulae) and is an external respiratory assistance device that takes venous blood, removes carbon dioxide and adds oxygen, much like a human lung. Oxygenated blood is then sent back to the patient. Each year, more than 20,000 patients receive ECMO therapy in the United States.
“This acquisition is a natural progression toward improving patient care,” said Matthew D. Bacchetta, M.D., associate professor, Department of Thoracic Surgery at Vanderbilt University. “Breethe’s compact and all-in-one technology aims for improved patient ambulation, which can improve outcomes and promote active rehabilitation for patients with cardiopulmonary diseases.”
Abiomed recognizes the need for ECMO therapy for patients in need of oxygenation and has supported approximately 10,000 ECMO plus Impella (ECPella) patients with cardiogenic shock over the past 10 years. In Japan, more than half of Impella patients receive ECPella for hemodynamic and oxygenation support.
Breethe’s product is a first-of-its kind, easy-to-use compact ECMO system with an integrated oxygen concentrator that eliminates the need for bulky oxygen tanks to promote easier patient ambulation. It has a novel design that is intuitive for health care providers to set up, manage, and monitor. Abiomed invested in Breethe in mid-2019. Breethe has applied for 510(k) clearance by the U.S. Food and Drug Administration (FDA).
Breethe’s founder and the Hales Distinguished Professor of Surgery at University of Maryland School of Medicine, Bartley Griffith, M.D., is a renowned leader in multiple areas of adult cardiac surgery including mechanical circulatory support. Dr. Griffith has collaborated with Abiomed for a number of years and served as the principal investigator of RECOVER I. With decades of experience, Dr. Griffith and his team designed the cutting-edge Breethe system to improve patient outcomes, improve quality of life and reduce total cost of care by changing the way oxygenation is delivered.
“Abiomed is the best positioned company to build on the legacy of what we started,” said Dr. Griffith. “I am confident that the addition of Breethe’s technology into Abiomed’s product portfolio will further enhance Abiomed’s ability to improve outcomes for their patients and serve a new patient population.”
This acquisition provides Abiomed with the opportunity to innovate traditional ECMO technology, focusing on patient ambulation and recovery from acute respiratory failure. For many patients in cardiogenic shock, Impella is the optimal technology because it unloads the left ventricle, perfuses end organs and allows the heart to rest and recover. Abiomed recognizes patients in cardiogenic shock may also need oxygenation. ECMO perfuses the end organs but does not unload the left ventricle, which increases the oxygen demand of the myocardium (heart muscle) in these patients. For patients in cardiogenic shock, Impella with ECMO (ECPella) work together to unload the heart and oxygenate the body.
“Breethe will integrate with Abiomed and our manufacturing, quality, sales, engineering and research capabilities, including Abiomed’s best-in-class Clinical Support Center,” said Michael R. Minogue, Abiomed’s chairman, president and CEO. “This acquisition aligns with our principles of leading in technology and innovation, putting patients first and striving to continually improve outcomes. Physicians have asked Abiomed to bring this technology into our portfolio because of our ability to support patients, teach best practices, and collect critical data for research. This ECMO technology will allow us to treat cardiogenic shock patients who are already being supported with Impella, add pediatric offerings and treat a new patient population with respiratory failure.”
Multiple studies support the association of ECPella therapy to improve outcomes for patients who are suffering from cardiogenic shock and require oxygenation. The European Journal of Heart Failure, ASAIO, and the Journal of the American College of Cardiology have published studies that examine a combined 4,126 patients and conclude use of ECPella was associated with increased survival rates, as compared to patients who were treated with ECMO only. In addition to higher survival, the study in the European Journal of Heart Failure by Pappalardo et al. found higher rates of heart recovery with ECPella use than with ECMO only (62 percent vs. 36 percent; p=0.048).
Terms of the acquisition are not being disclosed at this time.
The Impella 2.5 and Impella CP devices received FDA PMA (pre-market approval) to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0, Impella LD, and Impella 5.5 with Smart Assist are FDA-approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP is FDA-approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with more than 10 years of FDA studies, real world clinical data on more than 140,000 patients and more than 650 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5 with Smart Assist is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
Azadeh Laffafian Ph.d., medical device analyst at GlobalData, said, "As the number of COVID-19 cases surge, so does the demand for extracorporeal membrane oxygenation (ECMO) therapy. Through the acquisition of Breethe and its ECMO system, Abiomed is hoping to help fill this demand. While an increase in the supply of ECMO equipment is important in addressing COVID-19, other factors limiting the use of ECMO therapy include requirements for vast hospital resources and a team of experienced staff. Therefore, only critically ill patients who are expected to receive the most benefit and have the best chance of survival are considered. The ECMO system will be a natural addition to Abiomed's product portfolio going forward. ECMO therapy can be used in conjunction with Abiomed's Impella heart pumps to treat patients with cardiogenic shock."
Patients who require ECMO therapy have a severe and life-threatening illness that stops their lungs from working properly. The system is connected to patients through tubes (cannulae) and is an external respiratory assistance device that takes venous blood, removes carbon dioxide and adds oxygen, much like a human lung. Oxygenated blood is then sent back to the patient. Each year, more than 20,000 patients receive ECMO therapy in the United States.
“This acquisition is a natural progression toward improving patient care,” said Matthew D. Bacchetta, M.D., associate professor, Department of Thoracic Surgery at Vanderbilt University. “Breethe’s compact and all-in-one technology aims for improved patient ambulation, which can improve outcomes and promote active rehabilitation for patients with cardiopulmonary diseases.”
Abiomed recognizes the need for ECMO therapy for patients in need of oxygenation and has supported approximately 10,000 ECMO plus Impella (ECPella) patients with cardiogenic shock over the past 10 years. In Japan, more than half of Impella patients receive ECPella for hemodynamic and oxygenation support.
Breethe’s product is a first-of-its kind, easy-to-use compact ECMO system with an integrated oxygen concentrator that eliminates the need for bulky oxygen tanks to promote easier patient ambulation. It has a novel design that is intuitive for health care providers to set up, manage, and monitor. Abiomed invested in Breethe in mid-2019. Breethe has applied for 510(k) clearance by the U.S. Food and Drug Administration (FDA).
Breethe’s founder and the Hales Distinguished Professor of Surgery at University of Maryland School of Medicine, Bartley Griffith, M.D., is a renowned leader in multiple areas of adult cardiac surgery including mechanical circulatory support. Dr. Griffith has collaborated with Abiomed for a number of years and served as the principal investigator of RECOVER I. With decades of experience, Dr. Griffith and his team designed the cutting-edge Breethe system to improve patient outcomes, improve quality of life and reduce total cost of care by changing the way oxygenation is delivered.
“Abiomed is the best positioned company to build on the legacy of what we started,” said Dr. Griffith. “I am confident that the addition of Breethe’s technology into Abiomed’s product portfolio will further enhance Abiomed’s ability to improve outcomes for their patients and serve a new patient population.”
This acquisition provides Abiomed with the opportunity to innovate traditional ECMO technology, focusing on patient ambulation and recovery from acute respiratory failure. For many patients in cardiogenic shock, Impella is the optimal technology because it unloads the left ventricle, perfuses end organs and allows the heart to rest and recover. Abiomed recognizes patients in cardiogenic shock may also need oxygenation. ECMO perfuses the end organs but does not unload the left ventricle, which increases the oxygen demand of the myocardium (heart muscle) in these patients. For patients in cardiogenic shock, Impella with ECMO (ECPella) work together to unload the heart and oxygenate the body.
“Breethe will integrate with Abiomed and our manufacturing, quality, sales, engineering and research capabilities, including Abiomed’s best-in-class Clinical Support Center,” said Michael R. Minogue, Abiomed’s chairman, president and CEO. “This acquisition aligns with our principles of leading in technology and innovation, putting patients first and striving to continually improve outcomes. Physicians have asked Abiomed to bring this technology into our portfolio because of our ability to support patients, teach best practices, and collect critical data for research. This ECMO technology will allow us to treat cardiogenic shock patients who are already being supported with Impella, add pediatric offerings and treat a new patient population with respiratory failure.”
Multiple studies support the association of ECPella therapy to improve outcomes for patients who are suffering from cardiogenic shock and require oxygenation. The European Journal of Heart Failure, ASAIO, and the Journal of the American College of Cardiology have published studies that examine a combined 4,126 patients and conclude use of ECPella was associated with increased survival rates, as compared to patients who were treated with ECMO only. In addition to higher survival, the study in the European Journal of Heart Failure by Pappalardo et al. found higher rates of heart recovery with ECPella use than with ECMO only (62 percent vs. 36 percent; p=0.048).
Terms of the acquisition are not being disclosed at this time.
The Impella 2.5 and Impella CP devices received FDA PMA (pre-market approval) to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0, Impella LD, and Impella 5.5 with Smart Assist are FDA-approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP is FDA-approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with more than 10 years of FDA studies, real world clinical data on more than 140,000 patients and more than 650 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5 with Smart Assist is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
Azadeh Laffafian Ph.d., medical device analyst at GlobalData, said, "As the number of COVID-19 cases surge, so does the demand for extracorporeal membrane oxygenation (ECMO) therapy. Through the acquisition of Breethe and its ECMO system, Abiomed is hoping to help fill this demand. While an increase in the supply of ECMO equipment is important in addressing COVID-19, other factors limiting the use of ECMO therapy include requirements for vast hospital resources and a team of experienced staff. Therefore, only critically ill patients who are expected to receive the most benefit and have the best chance of survival are considered. The ECMO system will be a natural addition to Abiomed's product portfolio going forward. ECMO therapy can be used in conjunction with Abiomed's Impella heart pumps to treat patients with cardiogenic shock."