Business Wire08.07.19
PrinterPrezz Inc., a trailblazer in combining metal 3D printing, nanotechnologies and surgical expertise to design and manufacture next generation medical devices, has achieved ISO 13485:2016 certification for Quality Management Systems for medical devices.
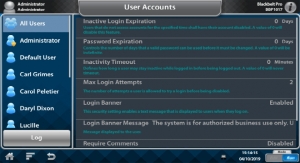
ISO 13485:2016 certification is a requirement for regulatory purposes and is an international standard that outlines the requirements for a quality management system specific to the medical devices industry. To be certified, organizations must demonstrate an ability to provide medical devices and related services that consistently meet customer and regulatory requirements. PrinterPrezz has met these standards for fabrication and 3D printing of titanium, non-titanium metals and advanced resin and plastic non-active implantable and non-implantable medical devices, associated components and surgical tools for the medical device industry.
Shri Shetty, CEO of PrinterPrezz, said, “Designing and implementing a quality management system and achieving ISO 13485:2016 certification are important milestones for our company that reflect our emphasis on safety and providing high-quality devices. I am proud of the collaborative efforts of our team which underscore our commitment to supporting our customers, as together we revolutionize the way advanced medical devices are developed and brought to market.”
PrinterPrezz’s mission is to bring more ideas for innovative medical devices to market faster, connecting medicine and manufacturing to become the first Medifacturing company in the world. By developing advanced medical devices using processes that combine expertise in 3D printing, orthopedics, semiconductor and nanotechnologies, PrinterPrezz’s ultimate goal is to provide medical solutions that enable people to enjoy active lives longer. PrinterPrezz’s ecosystem aims to solve challenges for various parts of the medical innovation value chain by providing prototyping, development, and manufacturing services to create life-enhancing medical devices. PrinterPrezz provides customers with access to a variety of 3D printing machines, 3D manipulation software, and 3D scanners as well as advanced manufacturing processes, and surgeon education programs. Medical solutions created by PrinterPrezz are designed to enable more people to live happier and more gratifying lives.
ISO 13485:2016 certification is a requirement for regulatory purposes and is an international standard that outlines the requirements for a quality management system specific to the medical devices industry. To be certified, organizations must demonstrate an ability to provide medical devices and related services that consistently meet customer and regulatory requirements. PrinterPrezz has met these standards for fabrication and 3D printing of titanium, non-titanium metals and advanced resin and plastic non-active implantable and non-implantable medical devices, associated components and surgical tools for the medical device industry.
Shri Shetty, CEO of PrinterPrezz, said, “Designing and implementing a quality management system and achieving ISO 13485:2016 certification are important milestones for our company that reflect our emphasis on safety and providing high-quality devices. I am proud of the collaborative efforts of our team which underscore our commitment to supporting our customers, as together we revolutionize the way advanced medical devices are developed and brought to market.”
PrinterPrezz’s mission is to bring more ideas for innovative medical devices to market faster, connecting medicine and manufacturing to become the first Medifacturing company in the world. By developing advanced medical devices using processes that combine expertise in 3D printing, orthopedics, semiconductor and nanotechnologies, PrinterPrezz’s ultimate goal is to provide medical solutions that enable people to enjoy active lives longer. PrinterPrezz’s ecosystem aims to solve challenges for various parts of the medical innovation value chain by providing prototyping, development, and manufacturing services to create life-enhancing medical devices. PrinterPrezz provides customers with access to a variety of 3D printing machines, 3D manipulation software, and 3D scanners as well as advanced manufacturing processes, and surgeon education programs. Medical solutions created by PrinterPrezz are designed to enable more people to live happier and more gratifying lives.