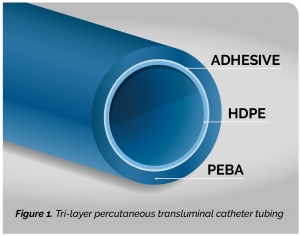
PR Newswire03.07.19
Biocoat Inc., a specialty manufacturer of hydrophilic biomaterial coatings for medical devices, has announced the company has received the certification of registration for the ISO 13485:2016 Quality Management System. The Horsham, Pa.-based company specializes in supplying lubricious hydrophilic coatings for medical devices used in the neurovascular, cardiovascular, peripheral, and ophthalmic markets to original equipment manufacturers and contract manufacturers.
This certification process reflects a culmination of Biocoat's ongoing commitment to quality during the production of medical-grade surface coatings for medical device applications, the manufacturing and distribution of medical devices used for sterile sperm selection, and an in-vitro diagnostic device for sperm analysis.
"Biocoat is committed to providing our customers with a best-in-class customer experience. We are dedicated to developing products of the highest quality while operating under a QMS that drives continuous improvement," said Jim Moran, president and CEO of Biocoat. "I am extremely proud of the Biocoat team and their dedication to ensuring that the highest levels of quality are being met every day."
To learn more about ISO 13485:2016, visit https://www.iso.org/standard/59752.html.
This certification process reflects a culmination of Biocoat's ongoing commitment to quality during the production of medical-grade surface coatings for medical device applications, the manufacturing and distribution of medical devices used for sterile sperm selection, and an in-vitro diagnostic device for sperm analysis.
"Biocoat is committed to providing our customers with a best-in-class customer experience. We are dedicated to developing products of the highest quality while operating under a QMS that drives continuous improvement," said Jim Moran, president and CEO of Biocoat. "I am extremely proud of the Biocoat team and their dedication to ensuring that the highest levels of quality are being met every day."
To learn more about ISO 13485:2016, visit https://www.iso.org/standard/59752.html.