Business Wire11.15.18
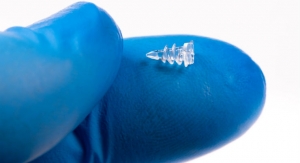
TransEnterix Inc., a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, has received U.S. Food and Drug Administration 510(k) clearance for 3 millimeter diameter instruments, as well as additional 5 millimeter Senhance System instruments.
The clearance of the 3 millimeter diameter instruments will allow the Senhance to be used for microlaparoscopic surgeries, enabling surgeons to operate through tiny incisions considered virtually scarless for patients.
“The ability to perform microlaparoscopic procedures using 3 millimeter instruments represents an unparalleled shift in the world of robotic surgery and a capability exclusive to the Senhance system,” said Todd M. Pope, TransEnterix CEO. “The addition of 3 millimeter instruments will allow many high volume surgeries to be performed with smaller incisions, which supports our mission of advancing minimally invasive surgical capabilities within digital laparoscopy.”
“Utilizing 3 millimeter micro instruments on a robotic system represents a new advancement in reducing the invasiveness of many surgeries,” said Dr. Steven D. McCarus, M.D., FACOG, chief of Gynecologic Surgery at Florida Hospital Celebration Health. “Patients find such small incisions to be virtually scarless and cosmetically desirable. Surgeons may find that using such tiny instruments with the precision and control of a digital interface makes microlaparoscopy a preferred option to treat more conditions.”
TransEnterix is a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options in today's value-based healthcare environment. The company is focused on the commercialization of the Senhance Surgical System, which digitizes laparoscopic minimally invasive surgery. The system allows for robotic precision, haptic feedback, surgeon camera control via eye sensing and improved ergonomics while offering responsible economics. The Senhance Surgical System is available for sale in the United States, the European Union, and select other countries.
The clearance of the 3 millimeter diameter instruments will allow the Senhance to be used for microlaparoscopic surgeries, enabling surgeons to operate through tiny incisions considered virtually scarless for patients.
“The ability to perform microlaparoscopic procedures using 3 millimeter instruments represents an unparalleled shift in the world of robotic surgery and a capability exclusive to the Senhance system,” said Todd M. Pope, TransEnterix CEO. “The addition of 3 millimeter instruments will allow many high volume surgeries to be performed with smaller incisions, which supports our mission of advancing minimally invasive surgical capabilities within digital laparoscopy.”
“Utilizing 3 millimeter micro instruments on a robotic system represents a new advancement in reducing the invasiveness of many surgeries,” said Dr. Steven D. McCarus, M.D., FACOG, chief of Gynecologic Surgery at Florida Hospital Celebration Health. “Patients find such small incisions to be virtually scarless and cosmetically desirable. Surgeons may find that using such tiny instruments with the precision and control of a digital interface makes microlaparoscopy a preferred option to treat more conditions.”
TransEnterix is a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options in today's value-based healthcare environment. The company is focused on the commercialization of the Senhance Surgical System, which digitizes laparoscopic minimally invasive surgery. The system allows for robotic precision, haptic feedback, surgeon camera control via eye sensing and improved ergonomics while offering responsible economics. The Senhance Surgical System is available for sale in the United States, the European Union, and select other countries.