Business Wire09.14.18
MedX Health Corp. announced that Dr. Trevor Champagne, a clinician in Quality and Innovation at Toronto’s Women's College Hospital, has joined its Medical Advisory Board. He is a board certified dermatologist in Canada and the United States.
“We are very pleased to have Dr. Champagne join our Medical Advisory Board as he brings unique skill sets, including expertise in Artificial Intelligence, virtual care and teledermatology, that are ideal in assisting MedX to more efficiently and quickly reach larger global markets,” said Scott Spearn, president of MedX. “Dr. Champagne has been using our SIAscopy technology and has already provided valuable input and clinician perspective into the telemedicine platform we are developing and releasing shortly.”
Champagne has a bachelor's degree in mathematics in computer science from the University of Waterloo and a B.Sc. in molecular biology and biochemistry from Simon Fraser University. He received his medical degree from the University of Western Ontario and is a graduate of the University of Toronto Dermatology residency program. He is a Lecturer in the Division of Dermatology at the University of Toronto.
"The ability for a health care technology to succeed at creating a viable artificial intelligence product depends on both the quality of the product and the company's ability to understand the safest, most effective way to use it. I think MedX has both,” said Champagne.
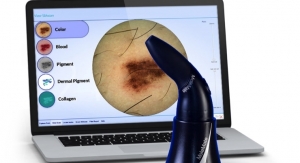
MedX, headquartered in Mississauga, Ontario, is a medical device company focused on skin cancer with its SIAscopy technology that is imbedded in its products SIAMETRICS, SIMSYS, and MoleMate, which MedX manufactures in its ISO 13485 certified facility. SIAMETRICS, SIMSYS, and MoleMate include hand-held devices that use patented technology utilizing light and its remittance to view up to 2 mm beneath suspicious moles and lesions in a pain free, non-invasive manner, with its software then creating real-time images for physicians and dermatologists to evaluate all types of moles or lesions within seconds. These products are Health Canada, U.S. Food and Drug Administration, ARTG and CE cleared for use in Canada, the United States, Australia, New Zealand, the European Union, and Turkey. MedX also designs, manufactures, and distributes quality laser and light therapy technologies to provide drug free and non-invasive treatment of tissue damage and pain.
“We are very pleased to have Dr. Champagne join our Medical Advisory Board as he brings unique skill sets, including expertise in Artificial Intelligence, virtual care and teledermatology, that are ideal in assisting MedX to more efficiently and quickly reach larger global markets,” said Scott Spearn, president of MedX. “Dr. Champagne has been using our SIAscopy technology and has already provided valuable input and clinician perspective into the telemedicine platform we are developing and releasing shortly.”
Champagne has a bachelor's degree in mathematics in computer science from the University of Waterloo and a B.Sc. in molecular biology and biochemistry from Simon Fraser University. He received his medical degree from the University of Western Ontario and is a graduate of the University of Toronto Dermatology residency program. He is a Lecturer in the Division of Dermatology at the University of Toronto.
"The ability for a health care technology to succeed at creating a viable artificial intelligence product depends on both the quality of the product and the company's ability to understand the safest, most effective way to use it. I think MedX has both,” said Champagne.
MedX, headquartered in Mississauga, Ontario, is a medical device company focused on skin cancer with its SIAscopy technology that is imbedded in its products SIAMETRICS, SIMSYS, and MoleMate, which MedX manufactures in its ISO 13485 certified facility. SIAMETRICS, SIMSYS, and MoleMate include hand-held devices that use patented technology utilizing light and its remittance to view up to 2 mm beneath suspicious moles and lesions in a pain free, non-invasive manner, with its software then creating real-time images for physicians and dermatologists to evaluate all types of moles or lesions within seconds. These products are Health Canada, U.S. Food and Drug Administration, ARTG and CE cleared for use in Canada, the United States, Australia, New Zealand, the European Union, and Turkey. MedX also designs, manufactures, and distributes quality laser and light therapy technologies to provide drug free and non-invasive treatment of tissue damage and pain.