Business Wire06.25.18
Auris Health Inc. has entered into a cooperative development and commercialization agreement with NeuWave Medical Inc., a subsidiary of Ethicon Inc., and part of the Johnson & Johnson Medical Devices Companies, to enable robotically assisted bronchoscopic ablation of lesions in the lung.
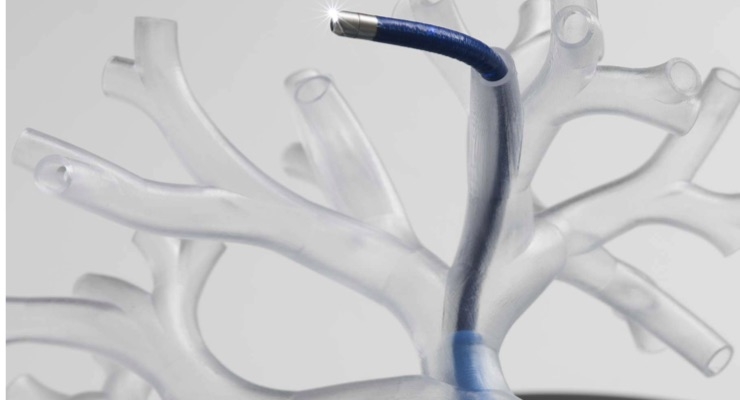
The agreement calls for co-development of integrated systems for robotic control, navigation, and application of microwave ablation delivered through bronchoscopes. A bronchoscope, equipped with a small camera and an accessory channel, allows tools to enter the lungs through the mouth. The co-development agreement also covers technology optimization and procedure development.
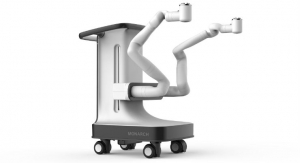
Auris Health’s Monarch platform, the first U.S. Food and Drug Administration (FDA)-cleared robotic platform for diagnostic and therapeutic bronchoscopic procedures, is designed to allow physicians to better diagnose small, hard-to-reach peripheral lung nodules. Ethicon’s NeuWave Flex Microwave Ablation System, the only FDA-cleared flexible microwave ablation probe, is an evolution of the NeuWave percutaneous microwave ablation system used throughout North America, Europe, and Asia. The new Flex system is indicated for soft tissue ablation in percutaneous (through an introducer) procedures, open surgical procedures, as well as in procedures in which the target tissue is accessed through a lumen or scope such as an endoscope.
“From the beginning, our vision has been to create a platform capable of enabling advanced diagnosis and therapy for a spectrum of disease, using the least invasive approach,” said Frederic Moll, M.D., co-founder and CEO of Auris Health. “We are honored to have Ethicon as a partner. Through this collaboration, we believe we are taking a significant first step together toward making the goal of diagnosing and treating lung cancer, all through the body’s natural openings, an eventual reality.”
“For those treating people with suspicious nodules in the lung, the holy grail is to one day be able to detect and treat the disease in a single procedure,” said Kazuhiro Yasufuku, M.D., associate professor of Surgery at the University of Toronto. “When this option becomes a reality, we may see many patients seek early screening and minimally invasive treatment.”
Lung cancer is the leading cause of cancer deaths worldwide. More patients die every year from the disease than from prostate, breast, and colon cancers combined.
Based in Silicon Valley, Auris Health is developing platforms that enhance physician capabilities, evolve minimally invasive techniques, and create new categories of care that redefine optimal patient outcomes. The company is integrating robotics, micro-instrumentation, endoscope design, sensing, and data science into one platform. Every element of its technology is driven by patient-specific design aimed at maintaining the integrity of the human body. The company is backed by Mithril Capital Management, Lux Capital, Coatue Management, and Highland Capital.
The agreement calls for co-development of integrated systems for robotic control, navigation, and application of microwave ablation delivered through bronchoscopes. A bronchoscope, equipped with a small camera and an accessory channel, allows tools to enter the lungs through the mouth. The co-development agreement also covers technology optimization and procedure development.
Auris Health’s Monarch platform, the first U.S. Food and Drug Administration (FDA)-cleared robotic platform for diagnostic and therapeutic bronchoscopic procedures, is designed to allow physicians to better diagnose small, hard-to-reach peripheral lung nodules. Ethicon’s NeuWave Flex Microwave Ablation System, the only FDA-cleared flexible microwave ablation probe, is an evolution of the NeuWave percutaneous microwave ablation system used throughout North America, Europe, and Asia. The new Flex system is indicated for soft tissue ablation in percutaneous (through an introducer) procedures, open surgical procedures, as well as in procedures in which the target tissue is accessed through a lumen or scope such as an endoscope.
“From the beginning, our vision has been to create a platform capable of enabling advanced diagnosis and therapy for a spectrum of disease, using the least invasive approach,” said Frederic Moll, M.D., co-founder and CEO of Auris Health. “We are honored to have Ethicon as a partner. Through this collaboration, we believe we are taking a significant first step together toward making the goal of diagnosing and treating lung cancer, all through the body’s natural openings, an eventual reality.”
“For those treating people with suspicious nodules in the lung, the holy grail is to one day be able to detect and treat the disease in a single procedure,” said Kazuhiro Yasufuku, M.D., associate professor of Surgery at the University of Toronto. “When this option becomes a reality, we may see many patients seek early screening and minimally invasive treatment.”
Lung cancer is the leading cause of cancer deaths worldwide. More patients die every year from the disease than from prostate, breast, and colon cancers combined.
Based in Silicon Valley, Auris Health is developing platforms that enhance physician capabilities, evolve minimally invasive techniques, and create new categories of care that redefine optimal patient outcomes. The company is integrating robotics, micro-instrumentation, endoscope design, sensing, and data science into one platform. Every element of its technology is driven by patient-specific design aimed at maintaining the integrity of the human body. The company is backed by Mithril Capital Management, Lux Capital, Coatue Management, and Highland Capital.