Business Wire07.09.18
MedX Health Corp. announced that Dr. Daniel Siegel, clinical professor of Dermatology at SUNY Downstate in Brooklyn, N.Y., and a former president of the American Academy of Dermatology, has joined its Medical Advisory Board.
“Daniel’s academic and practical business experience is impressive, including his expertise as a health policy and reimbursement specialist. His many skills will help MedX develop a strong launch platform for the U.S. market, where we see significant growth opportunities,” said Rob von der Porten, CEO of MedX.
Siegel received his medical degree from Albany Medical College in New York, and completed his residency in dermatology at Parkland Memorial Hospital in Dallas, Texas. He then pursued a fellowship in Mohs Micrographic Surgery and Dermatologic Surgery at Baylor College of Medicine in Houston, Texas. He also earned a master’s of science degree in management and policy from the W. Averell Harriman School for Management and Policy, State University of New York at Stony Brook.
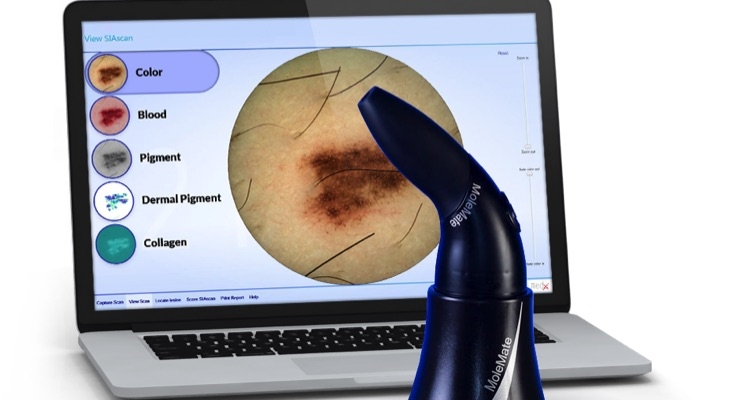
“I am very pleased to be joining MedX’s Medical Advisory Board and I look forward to assisting the company execute their plans for the U.S. market,” said Dr. Siegel. “MedX’s non-invasive SIAscope technology allows dermatologists to make accurate assessments of potential life-threatening melanomas because of their unique imaging technology.”
Siegel has published more than 100 articles and book chapters in major medical journals and textbooks. He is a reviewer, editor, and/or advisor for many of the major dermatology journals.
Siegel noted that “tele-dermatology has been slow to catch on because of its poor image quality, but with MedX’s clear dermascopic view combined with four additional views 2 mm below the skin’s surface, it’s as close to having the patient in front of you as I have seen.”
MedX, headquartered in Mississauga, Ontario, is a medical device company focused on skin cancer with its SIAscopy technology that is imbedded in its products SIAMETRICS, SIMSYS, and MoleMate, which MedX manufactures in its ISO 13485 certified facility. SIAMETRICS, SIMSYS, and MoleMate include handheld devices that use patented technology utilizing light and its remittance to view up to 2 mm beneath suspicious moles and lesions in a pain free, non-invasive manner, with its software then creating real-time images for physicians and dermatologists to evaluate all types of moles or lesions within seconds. These products are Health Canada-, U.S. Food and Drug Administration-, ARTG- and CE-cleared for use in Canada, the United States, Australia, New Zealand, the European Union, and Turkey. MedX also designs, manufactures, and distributes quality laser and light therapy technologies to provide drug free and non-invasive treatment of tissue damage and pain.
“Daniel’s academic and practical business experience is impressive, including his expertise as a health policy and reimbursement specialist. His many skills will help MedX develop a strong launch platform for the U.S. market, where we see significant growth opportunities,” said Rob von der Porten, CEO of MedX.
Siegel received his medical degree from Albany Medical College in New York, and completed his residency in dermatology at Parkland Memorial Hospital in Dallas, Texas. He then pursued a fellowship in Mohs Micrographic Surgery and Dermatologic Surgery at Baylor College of Medicine in Houston, Texas. He also earned a master’s of science degree in management and policy from the W. Averell Harriman School for Management and Policy, State University of New York at Stony Brook.
“I am very pleased to be joining MedX’s Medical Advisory Board and I look forward to assisting the company execute their plans for the U.S. market,” said Dr. Siegel. “MedX’s non-invasive SIAscope technology allows dermatologists to make accurate assessments of potential life-threatening melanomas because of their unique imaging technology.”
Siegel has published more than 100 articles and book chapters in major medical journals and textbooks. He is a reviewer, editor, and/or advisor for many of the major dermatology journals.
Siegel noted that “tele-dermatology has been slow to catch on because of its poor image quality, but with MedX’s clear dermascopic view combined with four additional views 2 mm below the skin’s surface, it’s as close to having the patient in front of you as I have seen.”
MedX, headquartered in Mississauga, Ontario, is a medical device company focused on skin cancer with its SIAscopy technology that is imbedded in its products SIAMETRICS, SIMSYS, and MoleMate, which MedX manufactures in its ISO 13485 certified facility. SIAMETRICS, SIMSYS, and MoleMate include handheld devices that use patented technology utilizing light and its remittance to view up to 2 mm beneath suspicious moles and lesions in a pain free, non-invasive manner, with its software then creating real-time images for physicians and dermatologists to evaluate all types of moles or lesions within seconds. These products are Health Canada-, U.S. Food and Drug Administration-, ARTG- and CE-cleared for use in Canada, the United States, Australia, New Zealand, the European Union, and Turkey. MedX also designs, manufactures, and distributes quality laser and light therapy technologies to provide drug free and non-invasive treatment of tissue damage and pain.