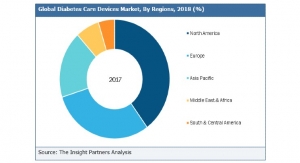
Globe Newswire08.03.18
Valeritas Holdings Inc., a medical technology company and maker of the V-Go Wearable Insulin Delivery device—a simple, all-in-one, wearable insulin delivery option for patients with diabetes—has announced positive results from the EffectiveNess of V-Go WeArable Insulin Delivery for Basal-BoLus ThErapy (ENABLE) Study.
The data show that patients who switched from insulin pens and syringes to V-Go significantly improved blood glucose while lowering insulin dose.
Valeritas CEO John Timberlake, commented, “The largest study of V-Go patients to date has demonstrated that regardless of baseline insulin dose or duration of diabetes, patients who switched to V-Go significantly lowered their blood glucose. We believe that is significant for diabetes patients, their medical professionals, payors, and Valeritas. We recently reported our third consecutive quarter of record revenue, which is a direct result of V-Go gaining momentum among patients with type 2 diabetes. V-Go is discreet, simple-to-use, cost-effective, clinically proven, and the only disposable, daily insulin delivery option.”
The first ENABLE Study analysis evaluated the clinical benefits in patients with type 2 diabetes who switched from using insulin pens and syringes to deliver their insulin regimen with V-Go for insulin delivery. The 283-patient, retrospective study demonstrated clinically and statistically significant reductions in A1C by over 1 percent (a measure of blood glucose control), and insulin total daily dose (TDD) at three and seven months. The percent of patients at high risk (A1C > 9 percent) was reduced by nearly 50 percent after switching to V-Go. In addition, over 50 percent of all patients in the study achieved an A1C less than 8 percent.
“We categorize patients with an A1C above 9 percent as high risk since they are more likely to have long-term complications that impact health care costs. The significant reduction in A1C and the achievement in A1C goals after switching to V-Go in the ENABLE study has important implications for treating diabetes. By improving glycemic control, our hope is to reduce rates of complications as well as decrease health care cost, which is a priority for all healthcare systems,” said lead author and endocrinologist Ripu Hundal, M.D., of First State Endocrinology in Newark, Del.
After three and seven months of V-Go use, significant reductions were observed in both A1C (-1.01 and -1.04; p<0.0001) and TDD (-17 U/day and -14 U/day; p<0.0001).
The second analysis from the ENABLE study confirmed V-Go provided a clinical benefit in a patient population poorly controlled on conventional basal-bolus therapy delivered by multiple daily injections (MDI) using insulin pens and syringes.
The analysis in 186 patients with type 2 diabetes demonstrated lowering of A1C by -1.0 at three months which was sustained at seven months and statistically significant with a P<0.0001. Insulin TDD was reduced by 30 percent (84 U/day to 59 U/day) with V-Go use. To determine if TDD was a factor in the change in A1C, patients were separated into three approximately equal groups based on baseline TDD (< 60 U/day [mean of 46 U/day], 60 to 90 U/day [mean of 72 U/day], or > 90 U/day [mean of 134 U/day]). Results demonstrated statistically significant A1C reductions across all TDD groups of -1.0, -0.8, -1.2, respectively, each with a P<0.001.
The third ENABLE study analysis evaluated the impact of duration of diabetes on change in A1C and insulin TDD when switching patients with type 2 diabetes from insulin delivery via insulin pen or syringe to V-Go. Patients with known duration of diabetes were stratified into five groups based on duration of diabetes (years). Clinically and statistically significant reductions in A1C from baseline at both three and seven-month intervals were observed in all five duration of diabetes strata. All strata also benefited from reductions in TDD with the exception of the duration stratum with the lowest baseline TDD (15 to 20 years), which maintained similar dosing on V-Go compared to baseline.
“Findings from the ENABLE Study demonstrated that no matter how long a patient had been diagnosed with diabetes, switching to V-Go from insulin delivery via insulin pens and syringes resulted in significantly lower A1C levels,” said lead author, Jane Cases, M.D., of Marietta, Ohio. “We believe this positive data demonstrates that diabetes duration should not be a factor when determining which patient can benefit from using V-Go.”
Valeritas is a commercial-stage medical technology company focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. Valeritas’ flagship product, V-Go Wearable Insulin Delivery device, is a simple, affordable, all-in-one basal-bolus insulin delivery option for patients with diabetes that is worn like a patch and can eliminate the need for taking multiple daily shots. V-Go administers a continuous preset basal rate of insulin over 24 hours, and it provides discreet on-demand bolus dosing at mealtimes. It is the only basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of type 2 diabetes patients. Headquartered in Bridgewater, N.J., Valeritas operates its R&D functions in Marlborough, Mass.
The data show that patients who switched from insulin pens and syringes to V-Go significantly improved blood glucose while lowering insulin dose.
Valeritas CEO John Timberlake, commented, “The largest study of V-Go patients to date has demonstrated that regardless of baseline insulin dose or duration of diabetes, patients who switched to V-Go significantly lowered their blood glucose. We believe that is significant for diabetes patients, their medical professionals, payors, and Valeritas. We recently reported our third consecutive quarter of record revenue, which is a direct result of V-Go gaining momentum among patients with type 2 diabetes. V-Go is discreet, simple-to-use, cost-effective, clinically proven, and the only disposable, daily insulin delivery option.”
The first ENABLE Study analysis evaluated the clinical benefits in patients with type 2 diabetes who switched from using insulin pens and syringes to deliver their insulin regimen with V-Go for insulin delivery. The 283-patient, retrospective study demonstrated clinically and statistically significant reductions in A1C by over 1 percent (a measure of blood glucose control), and insulin total daily dose (TDD) at three and seven months. The percent of patients at high risk (A1C > 9 percent) was reduced by nearly 50 percent after switching to V-Go. In addition, over 50 percent of all patients in the study achieved an A1C less than 8 percent.
“We categorize patients with an A1C above 9 percent as high risk since they are more likely to have long-term complications that impact health care costs. The significant reduction in A1C and the achievement in A1C goals after switching to V-Go in the ENABLE study has important implications for treating diabetes. By improving glycemic control, our hope is to reduce rates of complications as well as decrease health care cost, which is a priority for all healthcare systems,” said lead author and endocrinologist Ripu Hundal, M.D., of First State Endocrinology in Newark, Del.
After three and seven months of V-Go use, significant reductions were observed in both A1C (-1.01 and -1.04; p<0.0001) and TDD (-17 U/day and -14 U/day; p<0.0001).
The second analysis from the ENABLE study confirmed V-Go provided a clinical benefit in a patient population poorly controlled on conventional basal-bolus therapy delivered by multiple daily injections (MDI) using insulin pens and syringes.
The analysis in 186 patients with type 2 diabetes demonstrated lowering of A1C by -1.0 at three months which was sustained at seven months and statistically significant with a P<0.0001. Insulin TDD was reduced by 30 percent (84 U/day to 59 U/day) with V-Go use. To determine if TDD was a factor in the change in A1C, patients were separated into three approximately equal groups based on baseline TDD (< 60 U/day [mean of 46 U/day], 60 to 90 U/day [mean of 72 U/day], or > 90 U/day [mean of 134 U/day]). Results demonstrated statistically significant A1C reductions across all TDD groups of -1.0, -0.8, -1.2, respectively, each with a P<0.001.
The third ENABLE study analysis evaluated the impact of duration of diabetes on change in A1C and insulin TDD when switching patients with type 2 diabetes from insulin delivery via insulin pen or syringe to V-Go. Patients with known duration of diabetes were stratified into five groups based on duration of diabetes (years). Clinically and statistically significant reductions in A1C from baseline at both three and seven-month intervals were observed in all five duration of diabetes strata. All strata also benefited from reductions in TDD with the exception of the duration stratum with the lowest baseline TDD (15 to 20 years), which maintained similar dosing on V-Go compared to baseline.
“Findings from the ENABLE Study demonstrated that no matter how long a patient had been diagnosed with diabetes, switching to V-Go from insulin delivery via insulin pens and syringes resulted in significantly lower A1C levels,” said lead author, Jane Cases, M.D., of Marietta, Ohio. “We believe this positive data demonstrates that diabetes duration should not be a factor when determining which patient can benefit from using V-Go.”
Valeritas is a commercial-stage medical technology company focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. Valeritas’ flagship product, V-Go Wearable Insulin Delivery device, is a simple, affordable, all-in-one basal-bolus insulin delivery option for patients with diabetes that is worn like a patch and can eliminate the need for taking multiple daily shots. V-Go administers a continuous preset basal rate of insulin over 24 hours, and it provides discreet on-demand bolus dosing at mealtimes. It is the only basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of type 2 diabetes patients. Headquartered in Bridgewater, N.J., Valeritas operates its R&D functions in Marlborough, Mass.