Business Wire07.30.18
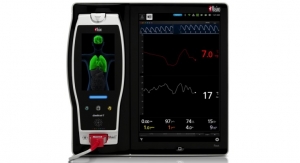
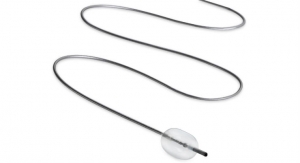
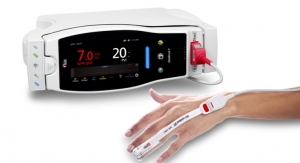
Masimo and Thermomedics, a PositiveID company, announced the debut and U.S. market release of TIR-1, a non-contact Bluetooth-enabled thermometer that integrates seamlessly with the Masimo Root patient monitoring and connectivity platform.
TIR-1 is an easy-to-use, clinical-grade infrared thermometer that provides forehead temperature measurements across all patient populations. As a non-contact device, the thermometer reduces the possibility of cross-contamination and eliminates the need to purchase additional disposables such as probe covers, reducing cost and waste. Its one-button operation delivers patient temperature results in seconds, reducing the time needed to take temperature measurements compared to traditional methods. Bluetooth connectivity, available exclusively in the customized thermometer developed by Thermomedics for Masimo, enables wireless data transfer to a connected Root monitor, automating efficient integration of an important patient vital sign into bedside devices, including early warning scores, and from there to hospital EMRs via Masimo Patient SafetyNet* or Iris Gateway.
Joe Kiani, founder and CEO of Masimo, said, “TIR-1 is a great example of the powerful expandability and versatility of the Root platform, and of our commitment to improving patient care through streamlined and automated workflows. This thermometer makes it easy to efficiently take the temperatures of multiple patients without having to manually record data. We’re delighted to have partnered with Thermomedics to develop an additional, easy-to-use thermometry option for Root customers.”
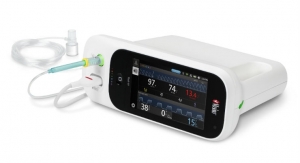
Masimo Root is an expandable patient monitoring and connectivity hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions—in a single, clinician-centric platform. Root’s plug-and-play expansion capabilities allow clinicians to centralize patient monitoring by bringing together advanced rainbow SET Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, and vital signs measurements—now including non-contact thermometry—on a customizable display, empowering clinicians with more information for making patient assessments.
William J. Caragol, chairman and CEO of PositiveID, said “Our team worked diligently to develop the TIR-1, the first Bluetooth-wireless non-contact thermometer to be paired with a vital signs monitoring system. We are very proud to bring this product to market with a company like Masimo and introduce non-contact thermometry to the Root platform.”
* The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
Thermomedics Inc. designs, develops and markets medical diagnostic equipment for professional healthcare providers. Its products are specifically designed for use in a wide range of medical settings, delivering nearly instantaneous diagnostic information. Thermomedics Inc. is a wholly owned subsidiary of PositiveID Corporation, a life sciences tools and diagnostics company. PositiveID specializes in the development of microfluidic systems in order to detect biological threats and outbreaks, whether airborne, in a healthcare setting, or at the point of need through its ExcitePCR subsidiary.
Masimo develops noninvasive monitoring technologies. In 1995, the company debuted Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), and more recently, Oxygen Reserve Index (ORi), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7 wearable patient monitor, iSpO2 pulse oximeter for smartphones, and the MightySat fingertip pulse oximeter.
ORi has not received U.S. Food and Drug Administration 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1.Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
TIR-1 is an easy-to-use, clinical-grade infrared thermometer that provides forehead temperature measurements across all patient populations. As a non-contact device, the thermometer reduces the possibility of cross-contamination and eliminates the need to purchase additional disposables such as probe covers, reducing cost and waste. Its one-button operation delivers patient temperature results in seconds, reducing the time needed to take temperature measurements compared to traditional methods. Bluetooth connectivity, available exclusively in the customized thermometer developed by Thermomedics for Masimo, enables wireless data transfer to a connected Root monitor, automating efficient integration of an important patient vital sign into bedside devices, including early warning scores, and from there to hospital EMRs via Masimo Patient SafetyNet* or Iris Gateway.
Joe Kiani, founder and CEO of Masimo, said, “TIR-1 is a great example of the powerful expandability and versatility of the Root platform, and of our commitment to improving patient care through streamlined and automated workflows. This thermometer makes it easy to efficiently take the temperatures of multiple patients without having to manually record data. We’re delighted to have partnered with Thermomedics to develop an additional, easy-to-use thermometry option for Root customers.”
Masimo Root is an expandable patient monitoring and connectivity hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions—in a single, clinician-centric platform. Root’s plug-and-play expansion capabilities allow clinicians to centralize patient monitoring by bringing together advanced rainbow SET Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, and vital signs measurements—now including non-contact thermometry—on a customizable display, empowering clinicians with more information for making patient assessments.
William J. Caragol, chairman and CEO of PositiveID, said “Our team worked diligently to develop the TIR-1, the first Bluetooth-wireless non-contact thermometer to be paired with a vital signs monitoring system. We are very proud to bring this product to market with a company like Masimo and introduce non-contact thermometry to the Root platform.”
* The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
Thermomedics Inc. designs, develops and markets medical diagnostic equipment for professional healthcare providers. Its products are specifically designed for use in a wide range of medical settings, delivering nearly instantaneous diagnostic information. Thermomedics Inc. is a wholly owned subsidiary of PositiveID Corporation, a life sciences tools and diagnostics company. PositiveID specializes in the development of microfluidic systems in order to detect biological threats and outbreaks, whether airborne, in a healthcare setting, or at the point of need through its ExcitePCR subsidiary.
Masimo develops noninvasive monitoring technologies. In 1995, the company debuted Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), and more recently, Oxygen Reserve Index (ORi), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7 wearable patient monitor, iSpO2 pulse oximeter for smartphones, and the MightySat fingertip pulse oximeter.
ORi has not received U.S. Food and Drug Administration 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1.Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.