Scott Gottlieb, M.D., Commissioner, U.S. Food and Drug Administration12.06.17
Once considered a futuristic technology on the distant horizon, 3D printing of medical devices, medications and human tissue is quickly becoming a promising reality. Patients have already benefitted from 3D printed medical products through access to personalized devices and innovative drugs that have led to significant health improvements. But the FDA is now preparing for a significant wave of new technologies that are nearly certain to transform medical practice. We’re working to provide a more comprehensive regulatory pathway that keeps pace with those advances, and helps facilitate efficient access to safe and effective innovations that are based on these technologies.
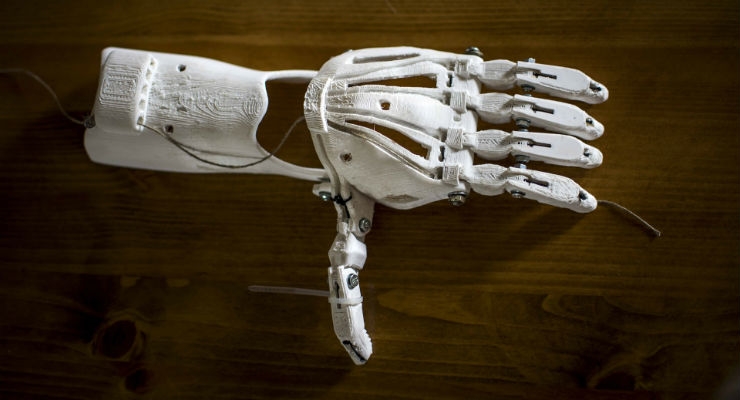
The FDA has reviewed more than 100 devices currently on the market that were manufactured on 3D printers. These include patient-matched devices tailored to fit a patient’s anatomy. Examples include knee replacements and implants designed to fit like a missing puzzle piece into a patient’s skull for facial reconstruction. We also approved the first drug produced on a 3D printer, which is used to treat seizures and has a more porous matrix than the drug manufactured in the traditional way, enabling the drug to dissolve more rapidly in the mouth to work faster. This is likely just the tip of the iceberg given the exponential growth of innovative research in this field. We envision that burn patients in the near future will be treated with their own new skin cells that are 3D printed directly onto their burn wounds. Further down the road, there is the potential for this same technology to eventually be used to develop replacement organs.
We’re also helping to advance the field of regulatory science with state-of-the-art 3D printing facilities located on the FDA’s campus. The Center for Drug Evaluation and Research’s (CDER) facility enables FDA scientists to conduct research to determine how the 3D printing of drugs impacts inactive ingredients and other drug components as well as the quality control process of manufacturing. FDA engineers in the Center for Devices and Radiological Health (CDRH) have been conducting research using their own 3D printing facility to investigate the effect of design changes on the safety and performance of devices, and to determine how iterative changes alter the device’s fit and functionality. Such answers could, for example, help improve the effectiveness and comfort of prosthetic devices. This research also helps inform us as regulators to help us understand the policy framework needed to ensure the quality and safety of 3D printed products.
To keep pace with evolving 3D printing technology and to encourage and support innovation in this field, the FDA has led the world in advancing efforts to provide a comprehensive policy framework to manufacturers and a more efficacious pathway to getting state-of-the-art medical products into the hands of patients and healthcare providers. One example has been CDER’s Emerging Technology Program, which provides opportunities for early engagement regarding innovative approaches to pharmaceutical product design or manufacturing, including additive manufacturing of pharmaceuticals. More than a dozen pharmaceutical manufacturers have formally or informally been in contact with CDER regarding the use of 3D printing to manufacture drugs.
Today we are issuing new guidance to help advise device manufacturers on technical aspects of 3D printing, referred to as additive manufacturing, that clarifies what the FDA recommends manufacturers include on submissions for 3D-printed medical devices. It includes our thinking on various approaches to 3D printing, including device design, testing of products for function and durability, and quality system requirements. Overall, it will help manufacturers bring their innovations to market more efficiently by providing a transparent process for future submissions and making sure our regulatory approach is properly tailored to the unique opportunities and challenges posed by this promising new technology.
But this technical guidance—categorized as a “leap-frog” guidance because it helps bridge where we are today with innovations of tomorrow—is only intended to provide the FDA’s initial thoughts on an emerging technology with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways. We are already seeing the beginning of this evolution as hospitals and academic centers use their own 3D printers to create innovative dental implants, replacement knee joints, and experimental heart valves and bone implants for use in clinical studies. An increasing number of surgeons across the country have been saving infants born with a life-threatening breathing condition by creating patient-matched 3D-printed splints to install in their patients’ tiny airways, which expand and degrade as the babies grow.
In order to help ensure the safety and effectiveness of these products, we’re working to establish a regulatory framework for how we plan to apply existing laws and regulations that govern device manufacturing to non-traditional manufacturers like medical facilities and academic institutions that create 3D-printed personalized devices for specific patients they are treating. Developing a transparent policy on 3D printing remains an important next step for us, and we plan to explore the role of nontraditional manufacturing facilities like a hospital operating room or university laboratory. The FDA also plans to review the regulatory issues related to the bioprinting of biological, cellular and tissue-based products in order to determine whether additional guidance is needed beyond the recently released regulatory framework on regenerative medicine medical products.The Center for Biologics Evaluation and Research has recently interacted with more than a half-dozen manufacturers who have expressed interest in using 3D printing in some capacity to produce their medical products.
These steps are part of our broader effort to help ensure our regulatory framework is properly matched to the unique attributes of the new technologies we’re being asked to review. 3D printing is certain to alter the daily practice of medicine where patients will be treated with medical products manufactured specifically for them. The FDA has an important mission to help advance these efforts while also protecting patients who depend on medical products to be safe and effective.
The FDA has reviewed more than 100 devices currently on the market that were manufactured on 3D printers. These include patient-matched devices tailored to fit a patient’s anatomy. Examples include knee replacements and implants designed to fit like a missing puzzle piece into a patient’s skull for facial reconstruction. We also approved the first drug produced on a 3D printer, which is used to treat seizures and has a more porous matrix than the drug manufactured in the traditional way, enabling the drug to dissolve more rapidly in the mouth to work faster. This is likely just the tip of the iceberg given the exponential growth of innovative research in this field. We envision that burn patients in the near future will be treated with their own new skin cells that are 3D printed directly onto their burn wounds. Further down the road, there is the potential for this same technology to eventually be used to develop replacement organs.
We’re also helping to advance the field of regulatory science with state-of-the-art 3D printing facilities located on the FDA’s campus. The Center for Drug Evaluation and Research’s (CDER) facility enables FDA scientists to conduct research to determine how the 3D printing of drugs impacts inactive ingredients and other drug components as well as the quality control process of manufacturing. FDA engineers in the Center for Devices and Radiological Health (CDRH) have been conducting research using their own 3D printing facility to investigate the effect of design changes on the safety and performance of devices, and to determine how iterative changes alter the device’s fit and functionality. Such answers could, for example, help improve the effectiveness and comfort of prosthetic devices. This research also helps inform us as regulators to help us understand the policy framework needed to ensure the quality and safety of 3D printed products.
To keep pace with evolving 3D printing technology and to encourage and support innovation in this field, the FDA has led the world in advancing efforts to provide a comprehensive policy framework to manufacturers and a more efficacious pathway to getting state-of-the-art medical products into the hands of patients and healthcare providers. One example has been CDER’s Emerging Technology Program, which provides opportunities for early engagement regarding innovative approaches to pharmaceutical product design or manufacturing, including additive manufacturing of pharmaceuticals. More than a dozen pharmaceutical manufacturers have formally or informally been in contact with CDER regarding the use of 3D printing to manufacture drugs.
Today we are issuing new guidance to help advise device manufacturers on technical aspects of 3D printing, referred to as additive manufacturing, that clarifies what the FDA recommends manufacturers include on submissions for 3D-printed medical devices. It includes our thinking on various approaches to 3D printing, including device design, testing of products for function and durability, and quality system requirements. Overall, it will help manufacturers bring their innovations to market more efficiently by providing a transparent process for future submissions and making sure our regulatory approach is properly tailored to the unique opportunities and challenges posed by this promising new technology.
But this technical guidance—categorized as a “leap-frog” guidance because it helps bridge where we are today with innovations of tomorrow—is only intended to provide the FDA’s initial thoughts on an emerging technology with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways. We are already seeing the beginning of this evolution as hospitals and academic centers use their own 3D printers to create innovative dental implants, replacement knee joints, and experimental heart valves and bone implants for use in clinical studies. An increasing number of surgeons across the country have been saving infants born with a life-threatening breathing condition by creating patient-matched 3D-printed splints to install in their patients’ tiny airways, which expand and degrade as the babies grow.
In order to help ensure the safety and effectiveness of these products, we’re working to establish a regulatory framework for how we plan to apply existing laws and regulations that govern device manufacturing to non-traditional manufacturers like medical facilities and academic institutions that create 3D-printed personalized devices for specific patients they are treating. Developing a transparent policy on 3D printing remains an important next step for us, and we plan to explore the role of nontraditional manufacturing facilities like a hospital operating room or university laboratory. The FDA also plans to review the regulatory issues related to the bioprinting of biological, cellular and tissue-based products in order to determine whether additional guidance is needed beyond the recently released regulatory framework on regenerative medicine medical products.The Center for Biologics Evaluation and Research has recently interacted with more than a half-dozen manufacturers who have expressed interest in using 3D printing in some capacity to produce their medical products.
These steps are part of our broader effort to help ensure our regulatory framework is properly matched to the unique attributes of the new technologies we’re being asked to review. 3D printing is certain to alter the daily practice of medicine where patients will be treated with medical products manufactured specifically for them. The FDA has an important mission to help advance these efforts while also protecting patients who depend on medical products to be safe and effective.