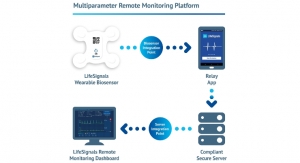
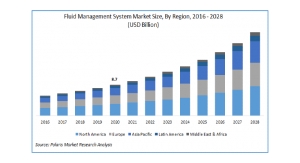
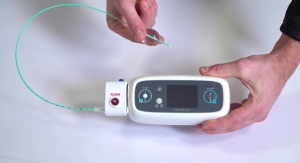
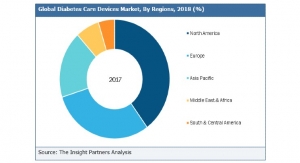
Cardinal Health11.30.17
Cordis, a Cardinal Health company, and Medinol announced United States Food and Drug Administration (FDA) approval of the EluNIR drug-eluting stent (DES) for the treatment of patients with narrowing or blockages to their coronary arteries. The EluNIR stent system is designed with a novel metallic spring tip and the narrowest strut width of any stent on the U.S. market to help clinicians easily deliver this new DES in highly complex anatomy and disease.
The EluNIR DES demonstrated outstanding efficacy and safety results in two randomized clinical trials, including BIONICS, a global pivotal study of 1,919 patients from 76 sites in eight countries. In BIONICS, the EluNIR stent demonstrated a 5.4 percent Target Lesion Failure (TLF), the lowest reported TLF in a contemporary U.S. pivotal study, and a zero percent rate of late stent thrombosis at 12 months. Medinol recently obtained CE-mark for the EluNIR stent, and it is currently being used by physicians in Europe.
"The BIONICS study demonstrated the excellent performance of the EluNIR DES in a broad, less selected, 'more comers' population," said David Kandzari, M.D., F.A.C.C., director of Interventional Cardiology at Piedmont Heart Institute in Atlanta, and principal investigator for the BIONICS trial. "Clinicians now have another safe, reliable option for treating the many patients whose lives are impacted by coronary artery disease."
Cardinal Health's long-term distribution agreement with Medinol enables Cordis, Cardinal Health's interventional vascular business, to sell Medinol's coronary stent portfolio, which now includes the EluNIR DES and NIRxcell, a cobalt-chromium bare metal stent (BMS), in the U.S.
"The FDA approval of the EluNIR stent expands the Cordis portfolio with a DES designed for deliverability, in even highly complex cases, with its novel stent and delivery system," said Luis Davila, vice president of R&D at Cordis. "Cordis is committed to ensuring physicians will soon have this new DES in their Cath Labs to deliver the best patient care available."
"Medinol has a legacy of developing innovative interventional cardiovascular technologies, which has culminated today with the FDA approval of the EluNIR DES," said Dr. Yoram Richter, chief scientific officer at Medinol. "For more than 20 years, Medinol has continuously raised the bar for the quality and performance of stenting systems. With our innovative manufacturing process, the EluNIR DES offers clinicians the latest generation DES. Notably, the FDA approval of the EluNIR DES marks the first such approval for a privately held company based outside of the U.S."
The EluNIR DES demonstrated outstanding efficacy and safety results in two randomized clinical trials, including BIONICS, a global pivotal study of 1,919 patients from 76 sites in eight countries. In BIONICS, the EluNIR stent demonstrated a 5.4 percent Target Lesion Failure (TLF), the lowest reported TLF in a contemporary U.S. pivotal study, and a zero percent rate of late stent thrombosis at 12 months. Medinol recently obtained CE-mark for the EluNIR stent, and it is currently being used by physicians in Europe.
"The BIONICS study demonstrated the excellent performance of the EluNIR DES in a broad, less selected, 'more comers' population," said David Kandzari, M.D., F.A.C.C., director of Interventional Cardiology at Piedmont Heart Institute in Atlanta, and principal investigator for the BIONICS trial. "Clinicians now have another safe, reliable option for treating the many patients whose lives are impacted by coronary artery disease."
Cardinal Health's long-term distribution agreement with Medinol enables Cordis, Cardinal Health's interventional vascular business, to sell Medinol's coronary stent portfolio, which now includes the EluNIR DES and NIRxcell, a cobalt-chromium bare metal stent (BMS), in the U.S.
"The FDA approval of the EluNIR stent expands the Cordis portfolio with a DES designed for deliverability, in even highly complex cases, with its novel stent and delivery system," said Luis Davila, vice president of R&D at Cordis. "Cordis is committed to ensuring physicians will soon have this new DES in their Cath Labs to deliver the best patient care available."
"Medinol has a legacy of developing innovative interventional cardiovascular technologies, which has culminated today with the FDA approval of the EluNIR DES," said Dr. Yoram Richter, chief scientific officer at Medinol. "For more than 20 years, Medinol has continuously raised the bar for the quality and performance of stenting systems. With our innovative manufacturing process, the EluNIR DES offers clinicians the latest generation DES. Notably, the FDA approval of the EluNIR DES marks the first such approval for a privately held company based outside of the U.S."