Business Wire09.28.17
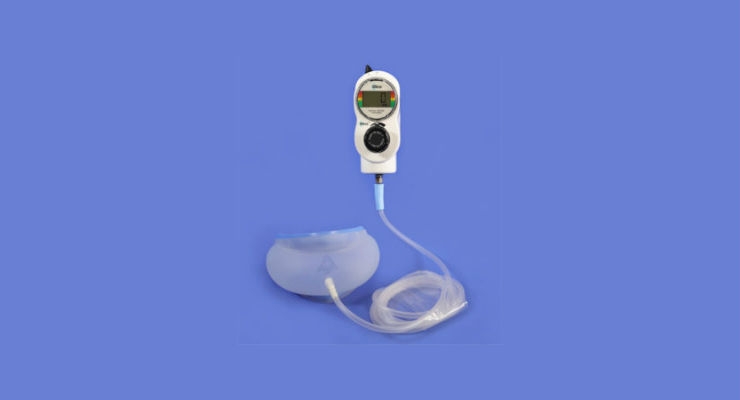
Smiths Medical, a global medical device manufacturer, has announced a distribution partnership with Sommetrics, a company that develops products and services to treat airway problems that arise during medical procedures and during sleep. As part of the partnership, Smiths Medical will distribute Sommetrics’ new aerFree Airway Management System (AMS), the first U.S. Food and Drug Administration-cleared external negative pressure aid that maintains a patent upper airway during mild to moderate sedation.
aerFree AMS is a non-invasive, safe and comfortable device intended to give clinicians a simple method of ensuring that a patient’s airway remains open during medical procedures while the patient is sedated. Smiths Medical will distribute aerFree AMS in select U.S. markets, followed by a global launch in 2018.
“The addition of the aerFree Airway Management System enhances our overall airway product portfolio and we are confident that this addition will add significant enhancements to patient care,” said Ian Harper, vice president of Vital Care at Smiths Medical.
“This distribution relationship with Smiths Medical enables a new and valuable technology to be quickly and efficiently introduced to the airway marketplace, where very few new product categories have been introduced in recent years,” said George Winston, general manager of Sommetrics Acute Care. “Clinician feedback at several professional conferences has clearly demonstrated the need for and excitement about the aerFree AMS as a new airway management tool.”
Smiths Medical is a supplier of specialized medical devices and equipment for global markets, focusing on the medication delivery, vital care and safety devices market segments. It is part of Smiths Group, a global developer of advanced technologies for markets in threat and contraband detection, energy, medical devices, communications and engineered components. Smiths Group employs around 23,000 people in more than 50 countries.
Sommetrics is a privately funded company in San Diego, Calif. It is primarily focused on improving sleep quality by providing products and services that deal with disorders of the upper airway such as obstructive sleep apnea and snoring. In addition, the company has developed products that address sedation-related compromise of the upper airway during medical procedures.
aerFree AMS is a non-invasive, safe and comfortable device intended to give clinicians a simple method of ensuring that a patient’s airway remains open during medical procedures while the patient is sedated. Smiths Medical will distribute aerFree AMS in select U.S. markets, followed by a global launch in 2018.
“The addition of the aerFree Airway Management System enhances our overall airway product portfolio and we are confident that this addition will add significant enhancements to patient care,” said Ian Harper, vice president of Vital Care at Smiths Medical.
“This distribution relationship with Smiths Medical enables a new and valuable technology to be quickly and efficiently introduced to the airway marketplace, where very few new product categories have been introduced in recent years,” said George Winston, general manager of Sommetrics Acute Care. “Clinician feedback at several professional conferences has clearly demonstrated the need for and excitement about the aerFree AMS as a new airway management tool.”
Smiths Medical is a supplier of specialized medical devices and equipment for global markets, focusing on the medication delivery, vital care and safety devices market segments. It is part of Smiths Group, a global developer of advanced technologies for markets in threat and contraband detection, energy, medical devices, communications and engineered components. Smiths Group employs around 23,000 people in more than 50 countries.
Sommetrics is a privately funded company in San Diego, Calif. It is primarily focused on improving sleep quality by providing products and services that deal with disorders of the upper airway such as obstructive sleep apnea and snoring. In addition, the company has developed products that address sedation-related compromise of the upper airway during medical procedures.