ClaroNav09.11.17
ClaroNav is pleased to announce that it has received FDA 510(k) clearance from the Food and Drug Administration (FDA) to market and sell NaviENT in the United States. Over the coming months the company will offer the product for sale to hospitals, clinics and offices performing endoscopic sinus surgery and skull base surgery in the U.S. NaviENT had previously been granted CE mark for the European market and Health Canada approval in 2016.
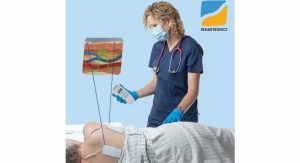
NaviENT is an Image-Guided Surgical navigation system, which helps ENT surgeons with identifying anatomic structures in the transnasal interventions. It enables surgeons to avoid complications and confidently make more informed decisions during FESS and skull base surgeries. NaviENT also results in cost saving by shortening length of hospitalization compared to non-navigated surgeries.
“Surgical Navigation system is a precious aid in primary surgery and its benefits are widely accepted among neurosurgeons and otolaryngologists. Its 3D anatomical structure localization and visualization capability assists the surgeons to target specific anatomy and avoid complications in highly variable sinus anatomy. However, despite its obvious advantages, many surgeons are hesitant in adapting the technology, blaming its cumbersome operation and high price tag.” said Ahmad Kolahi, CEO of CKI, a subsidiary of ClaroNav “NaviENT is an innovative navigation system designed in close collaboration with experienced rhinologists to address shortcomings of the current navigation systems. Our primary goal is to offer an intuitive, accurate and affordable state-of-the-art ENT navigation system and make it standard-of-care in the ENT field.”
Study shows that surgical navigation systems would allow more complete dissection, obviating the need for revision surgery. The American Academy of Otolargyngology – Head and Neck Surgery (AAO-HNS) endorses the use of image-guided surgery for many FESS and skull base procedures.
“NaviENT was designed in concert with rhinologists to develop the most user-friendly, accurate, reliable, and affordable navigation system on the market. It is clear that they have achieved their goals. In this case, using is believing.” said Peter Catalano, MD, FACS, FARS, professor of otolaryngology, chief of otolaryngology, St. Elizabeth’s Medical Center, Boston. “NaviENT is a most welcome addition for the ENT surgeon. This portable system provides quick set-up and accurate registration, a user-friendly interface, and versatile re-usable intra-operative tools. In addition, there are no disposable costs and the sale price is extremely affordable. NaviENT will effectively bring surgical navigation to every corner of the globe.”
For more information about the NaviENT, visit www.claronav.com/navient or the company’s booth at the following conferences:
NaviENT is an Image-Guided Surgical navigation system, which helps ENT surgeons with identifying anatomic structures in the transnasal interventions. It enables surgeons to avoid complications and confidently make more informed decisions during FESS and skull base surgeries. NaviENT also results in cost saving by shortening length of hospitalization compared to non-navigated surgeries.
“Surgical Navigation system is a precious aid in primary surgery and its benefits are widely accepted among neurosurgeons and otolaryngologists. Its 3D anatomical structure localization and visualization capability assists the surgeons to target specific anatomy and avoid complications in highly variable sinus anatomy. However, despite its obvious advantages, many surgeons are hesitant in adapting the technology, blaming its cumbersome operation and high price tag.” said Ahmad Kolahi, CEO of CKI, a subsidiary of ClaroNav “NaviENT is an innovative navigation system designed in close collaboration with experienced rhinologists to address shortcomings of the current navigation systems. Our primary goal is to offer an intuitive, accurate and affordable state-of-the-art ENT navigation system and make it standard-of-care in the ENT field.”
Study shows that surgical navigation systems would allow more complete dissection, obviating the need for revision surgery. The American Academy of Otolargyngology – Head and Neck Surgery (AAO-HNS) endorses the use of image-guided surgery for many FESS and skull base procedures.
“NaviENT was designed in concert with rhinologists to develop the most user-friendly, accurate, reliable, and affordable navigation system on the market. It is clear that they have achieved their goals. In this case, using is believing.” said Peter Catalano, MD, FACS, FARS, professor of otolaryngology, chief of otolaryngology, St. Elizabeth’s Medical Center, Boston. “NaviENT is a most welcome addition for the ENT surgeon. This portable system provides quick set-up and accurate registration, a user-friendly interface, and versatile re-usable intra-operative tools. In addition, there are no disposable costs and the sale price is extremely affordable. NaviENT will effectively bring surgical navigation to every corner of the globe.”
For more information about the NaviENT, visit www.claronav.com/navient or the company’s booth at the following conferences:
- ARS 63rd Annual Meeting, Sept 8 - 9, 2017 - Renaissance Chicago Downtown Hotel, Chicago, IL, Booth #23
- AAO-HNSF Annual Meeting, Sept 10 - 12, 2017, McCormick Place, Chicago, IL - Practice of the Future Pavilion - Booth OR2
- North American Masterclass in Endoscopic Sinus Surgery, Sept 14-17, 2017, McGill University, Montreal, Canada
- European ORL-HNS, Oct 7 - 11, 2017 - Barcelona, Spain
- MEDICA 2017, Nov 13 - 16, 2017 - Düsseldorf, Germany – Booth H10D07