U.S. Food and Drug Administration08.07.17
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death
Recalled Product
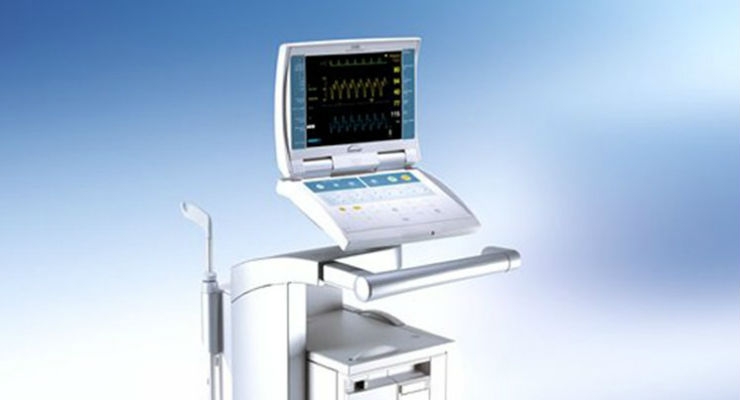
Datascope Corp./MAQUET's CS100i, CS100, and CS300 Intra-Aortic Balloon Pumps (IABP) are cardiac assist devices used to assist patients undergoing cardiac and non-cardiac surgery, and to treat patients with acute coronary syndrome or complications from heart failure.
Datascope Corp./MAQUET is recalling its CS100i, CS100, and CS300 Intra-Aortic Balloon Pumps manufactured before June 30, 2013 due to the risk of a valve failure which prevents the balloon from inflating and deflating properly. If a patient requires circulatory support with an IABP and the device does not work, or if therapy is stopped during use without a replacement IABP available, device failure may result in immediate and serious adverse health consequences, including death.
On June 30, 2013, Datascope Corp./MAQUET implemented a design change to prevent this problem, but not all devices manufactured prior to June 13, 2013 have been serviced and upgraded.
Who May Be Affected
On June 19, 2017, Datascope Corp./MAQUET sent affected customers an "Urgent Product Recall Medical Device Field Correction" notice informing them of the device's risks, and listing actions that should be taken to minimize the risk of patient harm until affected IABP units can be serviced. The firm recommends that the risks and benefits of using an affected CS100i, CS100, or CS300 IABP be assessed by the medical team for each patient when no alternative IABP or alternative therapy is available.
The notice directed customers to please adhere to the following instructions when using affected devices:
Customers with questions regarding this field correction may contact Datascope Corp./MAQUET's Customer Service Department at 1-(888)-627-8383 and press "2" (Monday through Friday from 8:00 a.m. to 6:00 p.m. EDT).
Recalled Product
- Datascope Corp./MAQUET Intra-Aortic Balloon Pump
- Model/Item Numbers: CS100i, CSO100, CS300
- Lot Numbers: All Lots Manufactured Before June 30, 2013
- Manufacturing Dates: July 22, 2003 to June 30, 2013
- Distribution Dates: March 24, 2003 to December 11, 2013
- Devices Recalled in the U.S.: 9,194
Datascope Corp./MAQUET's CS100i, CS100, and CS300 Intra-Aortic Balloon Pumps (IABP) are cardiac assist devices used to assist patients undergoing cardiac and non-cardiac surgery, and to treat patients with acute coronary syndrome or complications from heart failure.
Datascope Corp./MAQUET is recalling its CS100i, CS100, and CS300 Intra-Aortic Balloon Pumps manufactured before June 30, 2013 due to the risk of a valve failure which prevents the balloon from inflating and deflating properly. If a patient requires circulatory support with an IABP and the device does not work, or if therapy is stopped during use without a replacement IABP available, device failure may result in immediate and serious adverse health consequences, including death.
On June 30, 2013, Datascope Corp./MAQUET implemented a design change to prevent this problem, but not all devices manufactured prior to June 13, 2013 have been serviced and upgraded.
Who May Be Affected
- Hospitals and health care professionals using a Datascope Corp./MAQUET Intra-Aortic Balloon Pump that was manufactured before June 30, 2013 and has not been serviced and upgraded by the manufacturer.
- Patients receiving circulatory support with a Datascope Corp./MAQUET Intra-Aortic Balloon Pump that was manufactured before June 30, 2013.
On June 19, 2017, Datascope Corp./MAQUET sent affected customers an "Urgent Product Recall Medical Device Field Correction" notice informing them of the device's risks, and listing actions that should be taken to minimize the risk of patient harm until affected IABP units can be serviced. The firm recommends that the risks and benefits of using an affected CS100i, CS100, or CS300 IABP be assessed by the medical team for each patient when no alternative IABP or alternative therapy is available.
The notice directed customers to please adhere to the following instructions when using affected devices:
- Check inventory to identify any affected IABP units that may be stored or are currently in use.
- Pursuant to the User Instruction Warnings, health care providers are instructed not to leave a patient unattended during IABP therapy.
- A service representative from Datascope will be replacing the defective solenoid driver boards. Customers with affected IABP unit(s) will be contacted by a representative of the Maquet/Getinge Service Team to schedule on-site service.
- Until the service is performed, Datascope recommends powering on the IABP prior to inserting the IAB catheter to allow the IABP to successfully complete its self-test. This action will take less than 60 seconds to perform.
- In the event of a clinical emergency, health care providers should use a replacement IABP that has been serviced and upgraded, or an available alternative device.
- In the event the IABP fails to successfully complete the self-test and exhibits electrical test failure code 58, please remove the IABP from service and contact your local Maquet/Getinge Sales & Service Office.
- Customers are instructed to complete and return the Medical Device Field Correction Response Form enclosed in the notice via fax to 1-973-807-9217, or email at: IABP2017@getinge.com.
- If you are a distributor who has shipped any affected products to customers, please forward the Urgent Product Recall Medical Device Field Correction Notice to their attention for appropriate action.
Customers with questions regarding this field correction may contact Datascope Corp./MAQUET's Customer Service Department at 1-(888)-627-8383 and press "2" (Monday through Friday from 8:00 a.m. to 6:00 p.m. EDT).