PR Newswire05.31.16
Demand for real-time continuous data, including personal health information, early warning scores, and alerting when a patient's condition is deteriorating, is driving the market for complete monitoring systems in hospital and home care settings globally.
Isansys Lifecare, a provider of real-time patient data capture and analysis systems, has announced that demand for its complete, scalable, Patient Status Engine platforms has exceeded expectations as it has shipped systems to Norway, Germany, Scotland and India and is currently processing more orders for systems around the world.
Installation, including integration with a provider's IT system, has been designed to be simple and fast, usually completed within one day, thus allowing providers to deploy the system almost immediately and start collecting patient data. The objectives for each of the providers are to improve efficiencies and to provide accurate early warning indicators of adverse events without the additional labor costs associated with manual observations.
Dr. Line Pedersen, who is leading the work at Sorlandet Hospital Group, Norway where Isansys has just installed a number of systems, said, “This is an important project to improve patient safety. Through the use of the Isansys patient monitoring equipment across our hospital, I believe we can both reduce mortality and save money. We are looking forward to meeting our goal of delivering an exceptional patient care experience.”
"We are delighted to be deploying our systems to hospital and healthcare providers globally and that the value of this type of technology is now being recognized by people all around the world - the demand has completely surpassed our expectations. The PSE is an ideal match for a suite of healthcare solutions and will allow each of these countries to harness a wealth of new opportunities for improved clinical outcomes, reduced overall cost and increased revenues,” said Keith Errey, CEO of Isansys. "Our scalable and low-cost technology and service models offer clear benefits that make them uniquely suitable for these vast markets. We hope that by installing our systems in these hospitals and communities we can empower them to provide enhanced levels of proactive care.”
He added, "Automatic data capture addresses a whole range of issues relating to the pressures on nurses and greatly improves in-ward efficiencies compared with manual methods, including manual electronic observation systems. From any point of view, including cost, the PSE is very much the future of patient monitoring."
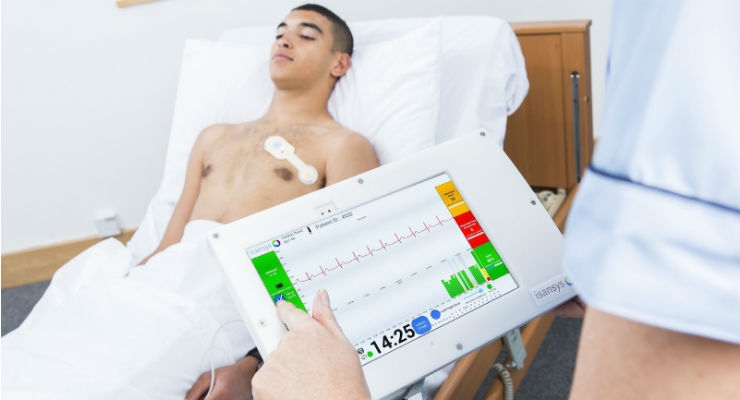
The PSE provides a complete end-to-end platform to continuously and wirelessly capture, collect, store and interpret vital sign and other physiological data. The Class IIA, CE-marked platform, uses the Lifetouch, Lifetemp, and other unobtrusive wireless, wearable sensors to collect data directly from the patient. The system analyses the ECG of every heartbeat to provide continuous heart rate, respiration rate, heart rate variability, and other cardiac parameters such as arrhythmias, and uses other sensors for continuous oxygen saturation, PPG, blood pressure, and temperature. This rich ensemble of physiological data is analyzed and simplified to allow doctors and nurses to monitor patients better, more closely, and more efficiently. It is clinically proven to assist in early detection of patient deterioration and helping to prevent adverse events.
The Patient Status Engine is both a medical device and a platform technology. The data that is collected can be used to address a range of clinical needs and is not focused on any one condition or disease. For instance, each country where the PSE has been shipped is using the platform to focus on different patient care pathway.
Isansys Lifecare, a provider of real-time patient data capture and analysis systems, has announced that demand for its complete, scalable, Patient Status Engine platforms has exceeded expectations as it has shipped systems to Norway, Germany, Scotland and India and is currently processing more orders for systems around the world.
Installation, including integration with a provider's IT system, has been designed to be simple and fast, usually completed within one day, thus allowing providers to deploy the system almost immediately and start collecting patient data. The objectives for each of the providers are to improve efficiencies and to provide accurate early warning indicators of adverse events without the additional labor costs associated with manual observations.
Dr. Line Pedersen, who is leading the work at Sorlandet Hospital Group, Norway where Isansys has just installed a number of systems, said, “This is an important project to improve patient safety. Through the use of the Isansys patient monitoring equipment across our hospital, I believe we can both reduce mortality and save money. We are looking forward to meeting our goal of delivering an exceptional patient care experience.”
"We are delighted to be deploying our systems to hospital and healthcare providers globally and that the value of this type of technology is now being recognized by people all around the world - the demand has completely surpassed our expectations. The PSE is an ideal match for a suite of healthcare solutions and will allow each of these countries to harness a wealth of new opportunities for improved clinical outcomes, reduced overall cost and increased revenues,” said Keith Errey, CEO of Isansys. "Our scalable and low-cost technology and service models offer clear benefits that make them uniquely suitable for these vast markets. We hope that by installing our systems in these hospitals and communities we can empower them to provide enhanced levels of proactive care.”
He added, "Automatic data capture addresses a whole range of issues relating to the pressures on nurses and greatly improves in-ward efficiencies compared with manual methods, including manual electronic observation systems. From any point of view, including cost, the PSE is very much the future of patient monitoring."
The PSE provides a complete end-to-end platform to continuously and wirelessly capture, collect, store and interpret vital sign and other physiological data. The Class IIA, CE-marked platform, uses the Lifetouch, Lifetemp, and other unobtrusive wireless, wearable sensors to collect data directly from the patient. The system analyses the ECG of every heartbeat to provide continuous heart rate, respiration rate, heart rate variability, and other cardiac parameters such as arrhythmias, and uses other sensors for continuous oxygen saturation, PPG, blood pressure, and temperature. This rich ensemble of physiological data is analyzed and simplified to allow doctors and nurses to monitor patients better, more closely, and more efficiently. It is clinically proven to assist in early detection of patient deterioration and helping to prevent adverse events.
The Patient Status Engine is both a medical device and a platform technology. The data that is collected can be used to address a range of clinical needs and is not focused on any one condition or disease. For instance, each country where the PSE has been shipped is using the platform to focus on different patient care pathway.
- In Norway the PSE is initially being used for automatic multiple vital sign data collection and analysis for early warning scores for early detection of patient deterioration
- In Germany, clinicians are focused on getting the patient out of hospital in the quickest time possible. This reduces the number of bed days for the patient and provides an opportunity for additional income for the hospital provider
- In Scotland clinicians will use the PSE to look for asymptomatic atrial fibrillation in a long term screening trial and in a separate project, to monitor premature babies. The PSE is the only wireless monitoring system approved for pediatric use.
- In India, Isansys is working with a number of leading hospital groups to provide early warning scores and other quantitative measures to improve patient outcomes, reduce costs for patient-payers, increase revenues for providers and provide out of hospital care.