BodyCap03.23.17
BodyCap, a company specialized in miniaturized wireless monitoring devices for e-health applications, has obtained CE Mark approval for e-Celsius, an ingestible connected pill that monitors the body’s core temperature. The device is now commercially available in all countries that recognize the CE mark (European Economic Area, Iceland, Liechtenstein and Norway). It will be sold directly or through specialized distributors.
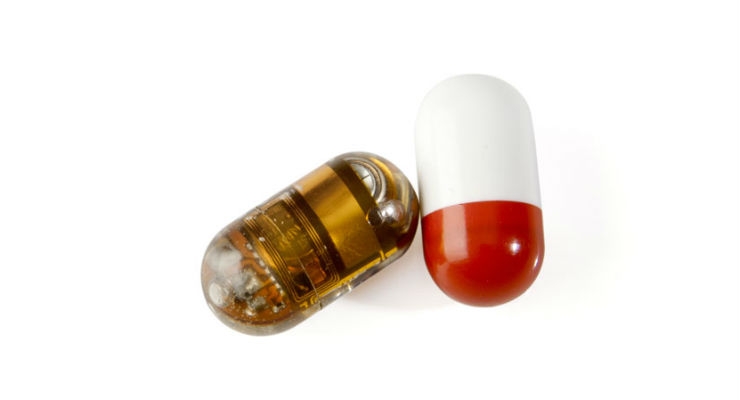
The e-Celsius product, a Class IIb medical device, allows continuous measurement of the patient’s central temperature by gastrointestinal tract. The disposable electronic capsule is coated in a biocompatible medical grade plastic. When swallowed by the patient it follows the intestinal transit. Every 30 seconds, the pill wirelessly transmits internal temperature measurements to a monitor called e-Viewer. This shows alerts when the measurement is outside the range set by the healthcare professional. The pill leaves the body naturally after one to three days.
The central temperature of the patient is one of the variable measures most regularly used during diagnosis or therapeutic follow-up in hospitals. It can detect an infectious peak, monitor the course of a fever or prevent the risk of hypothermia. Continuous monitoring of the patient's temperature is carried out in many situations:
The performance and reliability of the e-Celsius device have been validated in clinical trials. The studies demonstrate the heat homogeneity of the gastrointestinal tract and the equivalence with data from rectal and/or esophageal probes, currently considered to be gold standards within hospital settings. The tests have also shown that e-Celsius has a positive impact on patient and medical personnel well-being.
“The e-Celsius device is a true alternative method to the current use of rectal or eosophageal probes, which are invasive, uncomfortable, generate stress and limit the patient’s mobility,” said Sébastien Moussay, co-founder of BodyCap. “Our device is less intrusive and requires less from the medical staff, while at the same time increasing the well-being of both patients and healthcare personnel by lightening the workload. With the internal memory embedded in each capsule, e-Celsius ensures the monitoring of the patient's temperature kinetics in real time or deferred time, whatever the measurement conditions.”
The market for global ingestible smart pills should reach $1.47 billion (1.39 billion euros) by the end of 2024. e-Celsius integrates into a medical environment where reducing healthcare costs is a major issue. More than 230 million major surgeries are conducted worldwide annually. The development of ambulatory surgery to replace conventional surgery is a growing trend worldwide. BodyCap is a recognized player in the development of miniaturized sensors for health applications. The e-Celsius Performance device is already widely used in the field of elite sports and has been monitoring athletes since October 2015. It has been used during major events such as the Rio 2016 Olympics and the New York Marathon.
BodyCap develops miniaturized wireless electronic sensors and embedded solutions for health monitoring. Specialized in wearable connected devices for physiological data surveillance, its innovative high-tech products are used in sports performance enhancement, medical research and development and to monitor people in extreme environments. Its technologies have recently been used aboard the International Space Station (ISS) as part of the Proxima mission, in collaboration with the French Space National Agency.
BodyCap subscribes to a quality management process based on ISO13485 and ISO9001 standards. e-Celsius is a French-based manufacturing solution with partners LACROIX Electronics and ASICA. Its scientific board is made up of professors of medicine and pharmacology. The company works with global technological companies (DELL EMC, TOTEMSPARK) and research laboratories (INSERM, ESIEE). Fabrice Verjus, doctor of electronics, and Moussay, doctor of sports science, founded BodyCap in 2011. The company is based in Caen, France. It has an exclusive license for using a Philips patent and owns two other patents.
The e-Celsius product, a Class IIb medical device, allows continuous measurement of the patient’s central temperature by gastrointestinal tract. The disposable electronic capsule is coated in a biocompatible medical grade plastic. When swallowed by the patient it follows the intestinal transit. Every 30 seconds, the pill wirelessly transmits internal temperature measurements to a monitor called e-Viewer. This shows alerts when the measurement is outside the range set by the healthcare professional. The pill leaves the body naturally after one to three days.
The central temperature of the patient is one of the variable measures most regularly used during diagnosis or therapeutic follow-up in hospitals. It can detect an infectious peak, monitor the course of a fever or prevent the risk of hypothermia. Continuous monitoring of the patient's temperature is carried out in many situations:
- During major surgery, involving prolonged general anesthesia and post-operative follow-up
- During ambulatory surgery, for continuous temperature monitoring
- In general medicine, for diagnostic purposes
- In tropical medicine or infectiology, for faster management of pandemics
- While treating immunosuppressed patients or those at high risk of infection (sterile rooms)
- During follow-up of chemotherapy treatments, to better manage the side effects
- During sleep disorder analysis
The performance and reliability of the e-Celsius device have been validated in clinical trials. The studies demonstrate the heat homogeneity of the gastrointestinal tract and the equivalence with data from rectal and/or esophageal probes, currently considered to be gold standards within hospital settings. The tests have also shown that e-Celsius has a positive impact on patient and medical personnel well-being.
“The e-Celsius device is a true alternative method to the current use of rectal or eosophageal probes, which are invasive, uncomfortable, generate stress and limit the patient’s mobility,” said Sébastien Moussay, co-founder of BodyCap. “Our device is less intrusive and requires less from the medical staff, while at the same time increasing the well-being of both patients and healthcare personnel by lightening the workload. With the internal memory embedded in each capsule, e-Celsius ensures the monitoring of the patient's temperature kinetics in real time or deferred time, whatever the measurement conditions.”
The market for global ingestible smart pills should reach $1.47 billion (1.39 billion euros) by the end of 2024. e-Celsius integrates into a medical environment where reducing healthcare costs is a major issue. More than 230 million major surgeries are conducted worldwide annually. The development of ambulatory surgery to replace conventional surgery is a growing trend worldwide. BodyCap is a recognized player in the development of miniaturized sensors for health applications. The e-Celsius Performance device is already widely used in the field of elite sports and has been monitoring athletes since October 2015. It has been used during major events such as the Rio 2016 Olympics and the New York Marathon.
BodyCap develops miniaturized wireless electronic sensors and embedded solutions for health monitoring. Specialized in wearable connected devices for physiological data surveillance, its innovative high-tech products are used in sports performance enhancement, medical research and development and to monitor people in extreme environments. Its technologies have recently been used aboard the International Space Station (ISS) as part of the Proxima mission, in collaboration with the French Space National Agency.
BodyCap subscribes to a quality management process based on ISO13485 and ISO9001 standards. e-Celsius is a French-based manufacturing solution with partners LACROIX Electronics and ASICA. Its scientific board is made up of professors of medicine and pharmacology. The company works with global technological companies (DELL EMC, TOTEMSPARK) and research laboratories (INSERM, ESIEE). Fabrice Verjus, doctor of electronics, and Moussay, doctor of sports science, founded BodyCap in 2011. The company is based in Caen, France. It has an exclusive license for using a Philips patent and owns two other patents.