Mark Crawford, Contributing Writer11.04.19
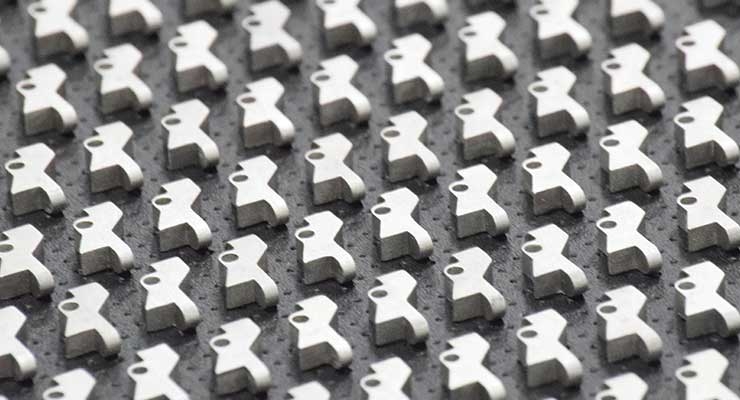
Rapid advances in additive manufacturing/3D printing (AM/3DP) have taken these technologies to the point where medical device companies can produce highly functional and durable metal and polymer components that deliver outstanding performance in the medical end-use environment. AM can simplify complex designs (fewer parts, fewer steps) and utilize new material configurations and alloys. AM-derived products can also comply with current requirements and regulations for testing and validation.
Because of these advances in equipment and materials, forward-thinking medical device manufacturers (MDMs) have made significant investments in additive manufacturing, intent on being first to market with creative new devices that cover a wide range of innovative applications. AM has seen the greatest acceptance in the orthopedic market, especially for creating implantable devices with porous or roughened surfaces that aid osseointegration, as well as disposable surgical guides and bone/anatomy models. Patient-specific implants are also being produced, using detailed imaging data. Spinal companies have also been quick to adopt these additive manufacturing techniques.
AM is perhaps the greatest driver of innovation in the medical device industry. The economics of AM have improved to the point where AM is more affordable and can be used to make a wider range of products, beyond the high-value, low-volume, niche applications they currently fabricate. The ability to produce intricate parts through AM, which cannot be made with traditional machining methods, has opened up huge opportunities for new applications, allowing engineers to think big and bold in the race to be first to market with innovative products.
“CEOs and senior executives of large companies are also openly pushing their vision of using 3D printing as a strategic growth engine throughout their organizations,” said Gautam Gupta, vice president of business development, healthcare, for 3D Systems, a Rock Hill, S.C.-based additive manufacturing solutions company. “This is unleashing innovation resulting in a large flux of new products entering the market.”
In fact, the rapidly growing demand for AM could, in the near future, create a shortage of high-quality AM manufacturers available to the medical device market. Brian R. McLaughlin, president and CEO of Amplify Additive, a Scarborough, Maine-based provider of additive manufacturing services, believes there are already not enough AM providers to go around. “AM is an underserved segment of the market, which is why we launched Amplify Additive,” he said. “There are simply not enough suppliers with enough know-how or equipment to provide the services needed by the medical device industry. The value proposition offered by additive manufacturing is simply too great to ignore—as a result, most ortho and medical companies are developing strategies to address their shortcomings with regards to understanding and leveraging AM.”
Latest AM Trends
Advantages of additive manufacturing to MDMs include mass customization, low cost in low volumes, expanded design freedom, and on-demand manufacturing. In addition to making complex implants, prosthetics, surgical instruments, and other devices, AM also enhances design and production processes by making prototypes and fixtures and other parts that are critical to streamlining production.
For example, Custom Mold and Design, a Forest Lake, Minn.-based provider of custom injection molds, engineering and design services, and precision machined components, has utilized AD/3DP to manufacture fixtures and prototypes for years. It also overmolds AM-produced titanium inserts with polyetheretherketone (PEEK) for spinal implants. “We focus on finding creative ways to solve complex problems,” said Lester Jones, chief operating officer for Custom Mold and Design. “Additive manufacturing, especially hybrid machine technology, is very helpful in making conformal-cooled inserts that incorporate complex curved, shaped, or spiral cooling channels.”
AM/3DP is being adopted beyond prototyping to include “higher-volume, final-product production,” said Pamela Lee, senior product manager for OmniCure UV LED Curing Solutions, part of Excelitas Technologies Corporation, a Waltham, Mass.-based provider of photonic detection and optical technologies. “Better quality and accuracy, faster speeds, and greater selection of materials have enhanced the capabilities of 3DP, while improving the economics to encourage more extensive adoption and increased accessibility.”
Metals are becoming easier to use in AM processes. AM that combines metal injection molding with sintering is beginning to gain traction for selected products. Steel parts with complicated shapes are good candidates for this approach, which also eliminates the need for expensive molds. “Other innovative applications involve manufacturing fixtures with direct metal laser sintering,” said Rachel Hunt, 3D printing marketing manager for Protolabs, a Maple Plain, Minn.-based provider of rapid prototyping and on-demand additive manufacturing services. “Our customers are starting to get creative with how they can utilize the technologies and resources available to them. Complex manufacturing fixtures that are not easy to machine can be manufactured with AM, significantly reducing labor time and cost.”
Much of the continued increase in AM capabilities is the result of software advances, which continue to happen at a rapid pace. These include image segmentation to convert patient image data to 3D models, as well as software to aid in topology optimization. “Over the last year I have noticed CAD plug-ins to create porous topologies for osseointegration have become more readily available,” said Chris Yakacki, associate professor of mechanical engineering at the University of Colorado-Boulder, who is deeply experienced in medical device manufacturing and has founded a biomedical startup. “For example, companies like nTopology have tools to automate a workflow for the optimization process. All you have to do is import a CAD file and the software will optimize and export the finished design very quickly.”
The successful adoption of any manufacturing process for a new medical device requires that the process meets all the OEM’s performance parameters, technical feasibility expectations, and economics established for the project—all of which involve speed. OEMs are laser-focused on speed of production and speed of delivery to market and, as a result, AM methods are getting faster all the time.
“With recent developments in AM, companies can take medical images and create models and patient-specific implants in a matter of days,” said Yakacki.
Speed can also be enhanced by selecting the right materials from a growing array of materials that are specifically engineered for AM. With newer resins that provide better mechanical properties and finishes, “the quality, speed, and nature of what can be manufactured has also evolved,” said Lee. “AM is providing greater scalability, more versatility, faster time to market, and higher levels of customizations. As print speeds continue to advance and costs come down, we will see more mass-market products adopt AM.”
What OEMs Want
Quality is at the top of every OEM list when it comes to AM/3DP. OEMs hold additive manufacturing to the same (or even higher) standards as traditional manufacturing methods. They want consistency, repeatability, and confidence in the process, with each part meeting the same required surface finish, dimensions, and functionality every time.
“In addition to quality, to even be considered by OEMs, contract manufacturers must be able to compete on AM cost,” said Matt Sand, president of 3DEO, a Los Angeles, Calif.-based provider of metal 3D printing services for a variety of industries, including medical. “If you are not in the ballpark on cost, you may as well not even play the game because OEMs are looking for the most cost-effective AM solution for their parts.”
Regarding features, OEMs are intent on using AM to create textured surfaces and structures for implants, such as hip and knee replacements and spinal fusion devices. “Component throughput and speed of print while maintaining highest quality are also important for making the business case viable,” said Stephen Anderson, additive manufacturing business manager for USA Renishaw, a West Dundee, Ill.-based engineering and scientific technology company that provides AM services. “These must also be coupled with the highest part quality, machine resolution, reliability, and repeatability. OEMs are also demanding streamlined post-processing.”
As devices become more complex and utilize advanced materials, OEMs seek manufacturing partners that can guide them through every stage of development, from design to production to regulatory approval. OEMs often request design-related services to help their engineers fully understand and adopt additive manufacturing, including guidance regarding material selection and application. “They are looking for established, validated platforms that can provide high reliability and simplified workflows to reduce the total cost of operation,” said Gupta. “Operational flexibility to scale quickly with shorter turnarounds when needed is also desired.”
OEMs not only want partners that are skilled in using AM technology and can help them with design, but “also who know how to deal with the secondary manufacturing processes for providing the finished part,” added McLaughlin. “Also, material handling for the powders used in the AM process is critical to the entire process and is certainly on the radar of the FDA.”
Many OEMs and contract manufacturers completely underestimate the regulatory requirements pertaining to AM and are not as familiar as they should be with FDA guidance documents. Contract manufacturers (CMs) are often asked by their clients to help support their 510(k) submissions. OEMs dread getting caught in the FDA pathway with a poorly substantiated submission. The FDA has provided solid guidance on what it expects from MDMs regarding validation of AM processes and products, and how to mitigate risk. CMs must be thoroughly familiar with the FDA guidance documents and help navigate their clients through the design and production process to minimize or eliminate any potential clashes with the FDA regarding their AM-derived devices.
Improved Hardware, Software, and Materials
AM equipment manufacturers continue to improve print speeds, reduce costs, enhance accuracy, expand the selection and range of hardware and software, and engineer new materials.
“The focus in hardware is on faster, more automated machines for shorter turnaround and reduced part cost,” said Gupta. “Incremental improvements such as standardized clamping systems are being included to seamlessly transition from additive to subtractive workflows.”
There is also a big push for higher overall speed, better surface finish, and in-situ monitoring that captures detailed information about the build. To meet these demands, manufacturers are building machines with multiple lasers and reduced layer height to shorten build time and help bring overall cost down for the AM part. Femtosecond lasers are being used more frequently to manage heat during the AM process. Superior gas flow is also essential to remove spatter and condensate from the build, which, if not removed, could cause build defects or act as defect origins and failure points for these components in the future. New machine designs also do a better job of separating the operator from physical interactions with the metal powder feedstock.
Innovations in software create smarter exposure strategies to minimize need of supports, thus reducing downstream machining efforts. Because of concerns about intellectual property (IP) protection, some machine manufacturers are incorporating software improvements that protect the IP exposure when files are shared with contract manufacturers worldwide for serial production. “I also see more requests for software add-ons that allow complete integration of the 3D printers into existing highly regulated production workflows within medical device companies,” said Gupta.
“Software advances will drive much of the industry growth,” added McLaughlin. “For example, generative design tools that allow the design of components based on load considerations will change the way we design, while leveraging the efficiency of AM.”
Over the years, materials science has made dramatic advances in AM-compatible materials, which include plastics, polymers, resins, ceramics, metals, alloys, composites, and biocompatibles. Materials can be customized to meet demanding characteristics for specific applications. Validated printing parameters have expanded to support 3D printing of implants and instruments in titanium, stainless steel (17-4 PH, 316L, etc.), and cobalt-chrome. “There is high interest in being able to print in PEEK, especially for spine and craniomaxillofacial applications, but this has proven to be a challenge so far,” said Gupta. “For non-implantable materials, there is a strong push toward biocompatible materials, materials that can be printed in multiple colors, and materials that are strong and can be sterilized for use within the operating room.”
Polymers continue to advance, with considerable R&D being spent on biologically functional polymers. These compounds will likely be biodegradable and also elicit specific biological responses when implanted. “Many of the synthetic methods to create these polymers can be readily incorporated into custom 3D printers at a reasonable price—about $20,000 or less,” said Yakacki.
Yakacki’s lab is using 3D printing to create soft polymers that mimic the behavior of tissues. He uses a direct ink writing process, which is similar to fused filament fabrication (FFF) or fused deposition modeling (FDM) printing, but also cures the polymer as it is being extruded. “This allows us to impart alignment within the polymer network that can mimic the alignment of collagen fibers,” said Yakacki. “The liquid-crystal polymer systems we use provide a great deal of dampening and our goal is to create artificial intervertebral disc and cartilage replacements. It would be much more difficult to control the structure of the polymer using conventional manufacturing techniques.”
Hybrid machines also exist that combine AM with traditional machining methods. Devices can be 3D-printed, followed by the machining out of certain features using standard equipment. Such machines are often built in-house to meet specific client needs.
“At our facility, we use a hybrid, powder bed metal additive manufacturing platform with subtractive machining capability,” said Jones. “This equipment uses metal powders that are melted and sintered using a laser, and then these surfaces are precisely milled at high speeds. This process allows for the production of the most complex and challenging parts through total manufacturing by digital engineering using 3D data.”
Another approach is 3DEO’s Intelligent Layering technology, which starts with a layer of metal powder that is sprayed with binder. The layer is then cut with micro-end mills to the shape of the part and this process is repeated until the part is complete. “While not technically a hybrid machine, this process combines the best of both 3D printing and computer numeric control [CNC] machining,” said Sand.
In order to make AM more efficient in the medical device space, resin suppliers, hardware manufacturers, and other players could collaborate more on materials. This would hopefully create a network where shared information allows MDMs to be more effective in their use of AM/3DP.
“We are excited by recent machine and material trends within the additive ecosystem that will further 3D printing adoption rates and motivate manufacturing engineering teams to consider AM earlier in the design for manufacturing process,” said Hunt.
Roy E. Morgan, vice president of engineering for Eagle Medical, a Paso Robles, Calif.-based provider of assembly, packaging, and sterilization services, also believes in collaboration to streamline the production process—especially regarding material selection.
“With any material, especially newer composites and alloys, the biggest challenge will always be biocompatibility,” said Morgan. “The material challenges specific to medical devices is calling industry toward a communal creation of a materials atlas that is similar to online applications and industry publications. Such an atlas would allow engineers and scientists to perform rapid screening of newer AM/3DP materials quickly to determine applicability and potential for success in design scenarios.”
OEM Misconceptions
With such rapid growth in AM features and capabilities, it is hard for OEMs to keep up with the latest developments; as a result, they may carry forward inaccurate assumptions or misunderstandings about what AM can or cannot do, often based on limited exposure in the past or outdated information. For example, many OEMs still see AM/3DP as only a rapid prototyping technology that is not capable of serial production, and therefore, they select traditional manufacturing over additive manufacturing, when AM may actually deliver better cost, volume, and quality.
“Most AM processes fail to achieve high volumes, repeatability, and high yields because they lack quality control systems that track and fix variability and degrading processes,” said Sand. “The AM/3DP industry lacks a standardized method of production like most traditional manufacturing methods have. However, at 3DEO, we can successfully achieve volumes in the thousands because we’ve designed our technology specifically for volume production and have taken measures in establishing process controls that ensure quality across the board.”
Companies are also not always aware of the other efficiencies that can be realized for instrument assemblies with 3D printing. “We have seen cases where engineers have used the technology to redesign an instrument assembly and reduced the number of components and the cost by more than half,” said Gupta. “The technology today can print in such high resolution that you can achieve acceptable surface finish for stainless steel instruments with minimal post processing. Of course, 3D printing is not the right technology for everything, but the speed and resolution of the technology is making it viable for many instrument applications.”
Another big misconception involves machine (printer) re-validation after a major component replacement such as a printer-head or laser mirror assembly. Installation qualification/operational qualification/performance qualification (IQ/OQ/PQ) may not be sufficient for re-validation. “To date, no detailed studies have been done on pre- and post-maintenance machine output to determine if such activities have significant impact on essential requirements of finished product,” said Morgan. “This will have to be addressed, especially as AM/3DP attempts to approach personalized medicine and batch sizes decrease.”
Moving Forward
AM technologies continue to advance on multiple fronts. Post-processing is a top focus, especially related to surface finish. Another is the development of test equipment to characterize AM powders. Ceramic AM is seeing more use for parts that are complicated in shape and/or may need patient-specific modifications. Anatomical printing is becoming more realistic, producing highly detailed models of anatomies and disease states.
“There is keen interest in the personalized medicine space,” said Morgan. “It offers great promise for providing unique AM solutions in metal and plastic. Even more fascinating are the recent developments in bio-printing that may enable creating of autologous tissue structures from a patient’s own stem cells.”
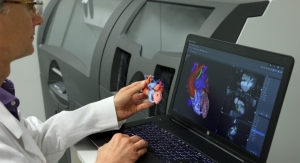
What is perhaps most exciting about AM is how data analytics can be used to improve device performance and patient outcomes. The clarity of data now available from various scanning modalities, especially computerized tomography and magnetic resonance imaging, is advancing rapidly and enabling highly detailed, patient-specific implants that can only be made with AM methods. This data, and the sophisticated tools that process it, are absolutely vital for improving the accuracy of surgical procedures and patient outcomes.
“A great example is a recent case at Great Ormond Street Hospital in London, U.K., where conjoined twins were recently and, more importantly, successfully separated,” said Anderson. “The surgical team used high-quality data to identify the anatomical structures of the individual twins to help plan the operation. Then, in a virtual setting, the team further planned how to rebuild their skulls.”
Anderson points out that it is easy to think of AM in isolation, but it is important to remember that it is part of a wider set of medical processes that, when used holistically, can make a huge difference in the quality of patient care. “As the understanding and skills within the imaging-3D printing-patient treatment loop develop and adoption increases, treatment costs will most likely drop, resulting in increased democratization of advanced care techniques for many more patients,” said Anderson.
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.
Because of these advances in equipment and materials, forward-thinking medical device manufacturers (MDMs) have made significant investments in additive manufacturing, intent on being first to market with creative new devices that cover a wide range of innovative applications. AM has seen the greatest acceptance in the orthopedic market, especially for creating implantable devices with porous or roughened surfaces that aid osseointegration, as well as disposable surgical guides and bone/anatomy models. Patient-specific implants are also being produced, using detailed imaging data. Spinal companies have also been quick to adopt these additive manufacturing techniques.
AM is perhaps the greatest driver of innovation in the medical device industry. The economics of AM have improved to the point where AM is more affordable and can be used to make a wider range of products, beyond the high-value, low-volume, niche applications they currently fabricate. The ability to produce intricate parts through AM, which cannot be made with traditional machining methods, has opened up huge opportunities for new applications, allowing engineers to think big and bold in the race to be first to market with innovative products.
“CEOs and senior executives of large companies are also openly pushing their vision of using 3D printing as a strategic growth engine throughout their organizations,” said Gautam Gupta, vice president of business development, healthcare, for 3D Systems, a Rock Hill, S.C.-based additive manufacturing solutions company. “This is unleashing innovation resulting in a large flux of new products entering the market.”
In fact, the rapidly growing demand for AM could, in the near future, create a shortage of high-quality AM manufacturers available to the medical device market. Brian R. McLaughlin, president and CEO of Amplify Additive, a Scarborough, Maine-based provider of additive manufacturing services, believes there are already not enough AM providers to go around. “AM is an underserved segment of the market, which is why we launched Amplify Additive,” he said. “There are simply not enough suppliers with enough know-how or equipment to provide the services needed by the medical device industry. The value proposition offered by additive manufacturing is simply too great to ignore—as a result, most ortho and medical companies are developing strategies to address their shortcomings with regards to understanding and leveraging AM.”
Latest AM Trends
Advantages of additive manufacturing to MDMs include mass customization, low cost in low volumes, expanded design freedom, and on-demand manufacturing. In addition to making complex implants, prosthetics, surgical instruments, and other devices, AM also enhances design and production processes by making prototypes and fixtures and other parts that are critical to streamlining production.
For example, Custom Mold and Design, a Forest Lake, Minn.-based provider of custom injection molds, engineering and design services, and precision machined components, has utilized AD/3DP to manufacture fixtures and prototypes for years. It also overmolds AM-produced titanium inserts with polyetheretherketone (PEEK) for spinal implants. “We focus on finding creative ways to solve complex problems,” said Lester Jones, chief operating officer for Custom Mold and Design. “Additive manufacturing, especially hybrid machine technology, is very helpful in making conformal-cooled inserts that incorporate complex curved, shaped, or spiral cooling channels.”
AM/3DP is being adopted beyond prototyping to include “higher-volume, final-product production,” said Pamela Lee, senior product manager for OmniCure UV LED Curing Solutions, part of Excelitas Technologies Corporation, a Waltham, Mass.-based provider of photonic detection and optical technologies. “Better quality and accuracy, faster speeds, and greater selection of materials have enhanced the capabilities of 3DP, while improving the economics to encourage more extensive adoption and increased accessibility.”
Metals are becoming easier to use in AM processes. AM that combines metal injection molding with sintering is beginning to gain traction for selected products. Steel parts with complicated shapes are good candidates for this approach, which also eliminates the need for expensive molds. “Other innovative applications involve manufacturing fixtures with direct metal laser sintering,” said Rachel Hunt, 3D printing marketing manager for Protolabs, a Maple Plain, Minn.-based provider of rapid prototyping and on-demand additive manufacturing services. “Our customers are starting to get creative with how they can utilize the technologies and resources available to them. Complex manufacturing fixtures that are not easy to machine can be manufactured with AM, significantly reducing labor time and cost.”
Much of the continued increase in AM capabilities is the result of software advances, which continue to happen at a rapid pace. These include image segmentation to convert patient image data to 3D models, as well as software to aid in topology optimization. “Over the last year I have noticed CAD plug-ins to create porous topologies for osseointegration have become more readily available,” said Chris Yakacki, associate professor of mechanical engineering at the University of Colorado-Boulder, who is deeply experienced in medical device manufacturing and has founded a biomedical startup. “For example, companies like nTopology have tools to automate a workflow for the optimization process. All you have to do is import a CAD file and the software will optimize and export the finished design very quickly.”
The successful adoption of any manufacturing process for a new medical device requires that the process meets all the OEM’s performance parameters, technical feasibility expectations, and economics established for the project—all of which involve speed. OEMs are laser-focused on speed of production and speed of delivery to market and, as a result, AM methods are getting faster all the time.
“With recent developments in AM, companies can take medical images and create models and patient-specific implants in a matter of days,” said Yakacki.
Speed can also be enhanced by selecting the right materials from a growing array of materials that are specifically engineered for AM. With newer resins that provide better mechanical properties and finishes, “the quality, speed, and nature of what can be manufactured has also evolved,” said Lee. “AM is providing greater scalability, more versatility, faster time to market, and higher levels of customizations. As print speeds continue to advance and costs come down, we will see more mass-market products adopt AM.”
What OEMs Want
Quality is at the top of every OEM list when it comes to AM/3DP. OEMs hold additive manufacturing to the same (or even higher) standards as traditional manufacturing methods. They want consistency, repeatability, and confidence in the process, with each part meeting the same required surface finish, dimensions, and functionality every time.
“In addition to quality, to even be considered by OEMs, contract manufacturers must be able to compete on AM cost,” said Matt Sand, president of 3DEO, a Los Angeles, Calif.-based provider of metal 3D printing services for a variety of industries, including medical. “If you are not in the ballpark on cost, you may as well not even play the game because OEMs are looking for the most cost-effective AM solution for their parts.”
Regarding features, OEMs are intent on using AM to create textured surfaces and structures for implants, such as hip and knee replacements and spinal fusion devices. “Component throughput and speed of print while maintaining highest quality are also important for making the business case viable,” said Stephen Anderson, additive manufacturing business manager for USA Renishaw, a West Dundee, Ill.-based engineering and scientific technology company that provides AM services. “These must also be coupled with the highest part quality, machine resolution, reliability, and repeatability. OEMs are also demanding streamlined post-processing.”
As devices become more complex and utilize advanced materials, OEMs seek manufacturing partners that can guide them through every stage of development, from design to production to regulatory approval. OEMs often request design-related services to help their engineers fully understand and adopt additive manufacturing, including guidance regarding material selection and application. “They are looking for established, validated platforms that can provide high reliability and simplified workflows to reduce the total cost of operation,” said Gupta. “Operational flexibility to scale quickly with shorter turnarounds when needed is also desired.”
OEMs not only want partners that are skilled in using AM technology and can help them with design, but “also who know how to deal with the secondary manufacturing processes for providing the finished part,” added McLaughlin. “Also, material handling for the powders used in the AM process is critical to the entire process and is certainly on the radar of the FDA.”
Many OEMs and contract manufacturers completely underestimate the regulatory requirements pertaining to AM and are not as familiar as they should be with FDA guidance documents. Contract manufacturers (CMs) are often asked by their clients to help support their 510(k) submissions. OEMs dread getting caught in the FDA pathway with a poorly substantiated submission. The FDA has provided solid guidance on what it expects from MDMs regarding validation of AM processes and products, and how to mitigate risk. CMs must be thoroughly familiar with the FDA guidance documents and help navigate their clients through the design and production process to minimize or eliminate any potential clashes with the FDA regarding their AM-derived devices.
Improved Hardware, Software, and Materials
AM equipment manufacturers continue to improve print speeds, reduce costs, enhance accuracy, expand the selection and range of hardware and software, and engineer new materials.
“The focus in hardware is on faster, more automated machines for shorter turnaround and reduced part cost,” said Gupta. “Incremental improvements such as standardized clamping systems are being included to seamlessly transition from additive to subtractive workflows.”
There is also a big push for higher overall speed, better surface finish, and in-situ monitoring that captures detailed information about the build. To meet these demands, manufacturers are building machines with multiple lasers and reduced layer height to shorten build time and help bring overall cost down for the AM part. Femtosecond lasers are being used more frequently to manage heat during the AM process. Superior gas flow is also essential to remove spatter and condensate from the build, which, if not removed, could cause build defects or act as defect origins and failure points for these components in the future. New machine designs also do a better job of separating the operator from physical interactions with the metal powder feedstock.
Innovations in software create smarter exposure strategies to minimize need of supports, thus reducing downstream machining efforts. Because of concerns about intellectual property (IP) protection, some machine manufacturers are incorporating software improvements that protect the IP exposure when files are shared with contract manufacturers worldwide for serial production. “I also see more requests for software add-ons that allow complete integration of the 3D printers into existing highly regulated production workflows within medical device companies,” said Gupta.
“Software advances will drive much of the industry growth,” added McLaughlin. “For example, generative design tools that allow the design of components based on load considerations will change the way we design, while leveraging the efficiency of AM.”
Over the years, materials science has made dramatic advances in AM-compatible materials, which include plastics, polymers, resins, ceramics, metals, alloys, composites, and biocompatibles. Materials can be customized to meet demanding characteristics for specific applications. Validated printing parameters have expanded to support 3D printing of implants and instruments in titanium, stainless steel (17-4 PH, 316L, etc.), and cobalt-chrome. “There is high interest in being able to print in PEEK, especially for spine and craniomaxillofacial applications, but this has proven to be a challenge so far,” said Gupta. “For non-implantable materials, there is a strong push toward biocompatible materials, materials that can be printed in multiple colors, and materials that are strong and can be sterilized for use within the operating room.”
Polymers continue to advance, with considerable R&D being spent on biologically functional polymers. These compounds will likely be biodegradable and also elicit specific biological responses when implanted. “Many of the synthetic methods to create these polymers can be readily incorporated into custom 3D printers at a reasonable price—about $20,000 or less,” said Yakacki.
Yakacki’s lab is using 3D printing to create soft polymers that mimic the behavior of tissues. He uses a direct ink writing process, which is similar to fused filament fabrication (FFF) or fused deposition modeling (FDM) printing, but also cures the polymer as it is being extruded. “This allows us to impart alignment within the polymer network that can mimic the alignment of collagen fibers,” said Yakacki. “The liquid-crystal polymer systems we use provide a great deal of dampening and our goal is to create artificial intervertebral disc and cartilage replacements. It would be much more difficult to control the structure of the polymer using conventional manufacturing techniques.”
Hybrid machines also exist that combine AM with traditional machining methods. Devices can be 3D-printed, followed by the machining out of certain features using standard equipment. Such machines are often built in-house to meet specific client needs.
“At our facility, we use a hybrid, powder bed metal additive manufacturing platform with subtractive machining capability,” said Jones. “This equipment uses metal powders that are melted and sintered using a laser, and then these surfaces are precisely milled at high speeds. This process allows for the production of the most complex and challenging parts through total manufacturing by digital engineering using 3D data.”
Another approach is 3DEO’s Intelligent Layering technology, which starts with a layer of metal powder that is sprayed with binder. The layer is then cut with micro-end mills to the shape of the part and this process is repeated until the part is complete. “While not technically a hybrid machine, this process combines the best of both 3D printing and computer numeric control [CNC] machining,” said Sand.
In order to make AM more efficient in the medical device space, resin suppliers, hardware manufacturers, and other players could collaborate more on materials. This would hopefully create a network where shared information allows MDMs to be more effective in their use of AM/3DP.
“We are excited by recent machine and material trends within the additive ecosystem that will further 3D printing adoption rates and motivate manufacturing engineering teams to consider AM earlier in the design for manufacturing process,” said Hunt.
Roy E. Morgan, vice president of engineering for Eagle Medical, a Paso Robles, Calif.-based provider of assembly, packaging, and sterilization services, also believes in collaboration to streamline the production process—especially regarding material selection.
“With any material, especially newer composites and alloys, the biggest challenge will always be biocompatibility,” said Morgan. “The material challenges specific to medical devices is calling industry toward a communal creation of a materials atlas that is similar to online applications and industry publications. Such an atlas would allow engineers and scientists to perform rapid screening of newer AM/3DP materials quickly to determine applicability and potential for success in design scenarios.”
OEM Misconceptions
With such rapid growth in AM features and capabilities, it is hard for OEMs to keep up with the latest developments; as a result, they may carry forward inaccurate assumptions or misunderstandings about what AM can or cannot do, often based on limited exposure in the past or outdated information. For example, many OEMs still see AM/3DP as only a rapid prototyping technology that is not capable of serial production, and therefore, they select traditional manufacturing over additive manufacturing, when AM may actually deliver better cost, volume, and quality.
“Most AM processes fail to achieve high volumes, repeatability, and high yields because they lack quality control systems that track and fix variability and degrading processes,” said Sand. “The AM/3DP industry lacks a standardized method of production like most traditional manufacturing methods have. However, at 3DEO, we can successfully achieve volumes in the thousands because we’ve designed our technology specifically for volume production and have taken measures in establishing process controls that ensure quality across the board.”
Companies are also not always aware of the other efficiencies that can be realized for instrument assemblies with 3D printing. “We have seen cases where engineers have used the technology to redesign an instrument assembly and reduced the number of components and the cost by more than half,” said Gupta. “The technology today can print in such high resolution that you can achieve acceptable surface finish for stainless steel instruments with minimal post processing. Of course, 3D printing is not the right technology for everything, but the speed and resolution of the technology is making it viable for many instrument applications.”
Another big misconception involves machine (printer) re-validation after a major component replacement such as a printer-head or laser mirror assembly. Installation qualification/operational qualification/performance qualification (IQ/OQ/PQ) may not be sufficient for re-validation. “To date, no detailed studies have been done on pre- and post-maintenance machine output to determine if such activities have significant impact on essential requirements of finished product,” said Morgan. “This will have to be addressed, especially as AM/3DP attempts to approach personalized medicine and batch sizes decrease.”
Moving Forward
AM technologies continue to advance on multiple fronts. Post-processing is a top focus, especially related to surface finish. Another is the development of test equipment to characterize AM powders. Ceramic AM is seeing more use for parts that are complicated in shape and/or may need patient-specific modifications. Anatomical printing is becoming more realistic, producing highly detailed models of anatomies and disease states.
“There is keen interest in the personalized medicine space,” said Morgan. “It offers great promise for providing unique AM solutions in metal and plastic. Even more fascinating are the recent developments in bio-printing that may enable creating of autologous tissue structures from a patient’s own stem cells.”
What is perhaps most exciting about AM is how data analytics can be used to improve device performance and patient outcomes. The clarity of data now available from various scanning modalities, especially computerized tomography and magnetic resonance imaging, is advancing rapidly and enabling highly detailed, patient-specific implants that can only be made with AM methods. This data, and the sophisticated tools that process it, are absolutely vital for improving the accuracy of surgical procedures and patient outcomes.
“A great example is a recent case at Great Ormond Street Hospital in London, U.K., where conjoined twins were recently and, more importantly, successfully separated,” said Anderson. “The surgical team used high-quality data to identify the anatomical structures of the individual twins to help plan the operation. Then, in a virtual setting, the team further planned how to rebuild their skulls.”
Anderson points out that it is easy to think of AM in isolation, but it is important to remember that it is part of a wider set of medical processes that, when used holistically, can make a huge difference in the quality of patient care. “As the understanding and skills within the imaging-3D printing-patient treatment loop develop and adoption increases, treatment costs will most likely drop, resulting in increased democratization of advanced care techniques for many more patients,” said Anderson.
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.