Bryce G. Rutter, Ph.D., Founder & CEO, Metaphase Design Group Inc.11.04.19
Unlike medical products that just need to work, drug delivery devices must also consider intuitiveness, usability, and how it makes the user feel when operated.
Device manufacturers today predominately use a design strategy that begins with engineering development of a device platform. R&D explores a wide variety of mechanical design options for calking and triggering a device, which in turn dictate its overall size, layout, and the forces required to reliably function. Only after the new device design is complete will manufacturers begin the search for a pharmaceutical partner that wants to use the device for its drug. It’s not surprising this engineering-driven design strategy leads to unintuitive and difficult to use device designs, because the device has been designed before it’s known which drug—and therefore, what type of user—will operate the device. This R&D strategy overlooks fundamental human factors considerations that directly impact usability. They have forgotten about the user.
It’s all about the user.
A user-centered design strategy is driven by how people think, feel, and behave. Mechanical design solutions meeting user needs can only be developed after truly understanding the user. Good device design begins with accommodating the widest possible range in hand, body size, and strength—ideally, the 5th percentile female to the 95th percentile male.
People commonly draw from one of four types of grips depending on the task’s complexity and the forces required to operate a device. When strength and endurance are required, people naturally gravitate toward power grips that recruit the larger forearm muscles. In contrast, for high precision and control, people default to hi-dexterity bilateral and trilateral grips that recruit smaller, weaker finger muscles.
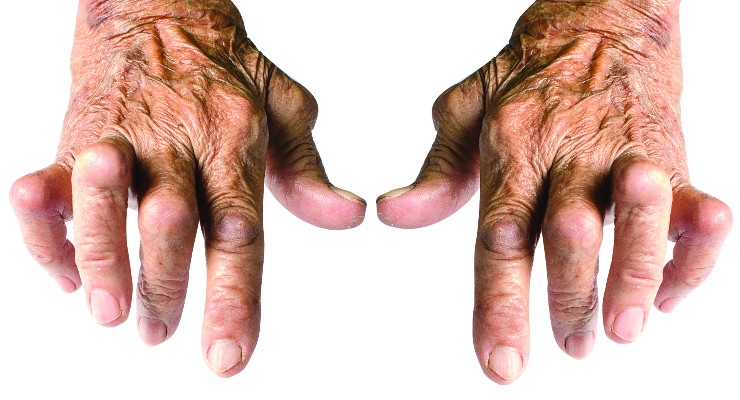
All of these common grips change when disease steps in and alters how people can use their hands. For example, developing devices for people with rheumatoid arthritis (RA) presents a unique design challenge. The disease causes severe joint pain in the fingers and wrists and diminishes strength and dexterity. Living with these impairments forces users to routinely modify grasping and handling behaviors to compensate.
Grip touchpoints also change because RA users commonly experience skewed knuckle joints that laterally shifts where the fingertips land on the device. Furthermore, the disease dramatically reduces strength and range of joint motion, which erode grasping and dexterity. It is not uncommon to see a 60-80 percent reduction in finger strength and 50 percent reduction in the range of joint motion. These factors define how to design specialized grips that meet RA users’ unique needs.
Pain is also a significant design factor in designing for arthritic hands. To reduce pain, industrial designers and human factors engineers can identify device sizes and shapes that encourage power grips engaging fewer finger joints and recruiting the stronger forearm muscles.
Connected to hand function are considerations for how different anatomic injection sites influence usability. Line of sight, power versus precision grasping, trigger actuation forces, and the ability to stabilize the device throughout injection play key roles in creating easy-to-use and intuitive designs. The need to properly align the device to the skin surface to ensure proper needle penetration depth for drug delivery is equally important. Evaluating the ergonomics of these design requirements will define the most effective grasping strategy as it relates to the anatomic site and placing the device perpendicular to the injection site for successful drug delivery.
Design for Dignity
We often hear about “dying with dignity,” but rarely about living with dignity. When becoming ill, we experience a loss of dignity because we often lose autonomy and feel vulnerable. It is common for those suffering from a chronic illness to question their self-worth and become depressed. Poor mental health and disease-related physical impairments directly impact patient compliance.
The right look and feel of a device can make a powerful emotional connection with users. A plethora of ugly medical devices projecting an unpleasant stigma exist in today’s market—these practically advertise the user’s illness or disability. Fundamental functional design oversights requiring awkward grasping patterns and triggers or actuators not located under the natural fall of the fingertip and frequently exceed the user’s strength exacerbate this stigma.
A poorly designed, unintuitive, hard-to-use product impacts how people feel about themselves. Struggling with a device is both frustrating and embarrassing. Bad designs create feelings of defeat, and many users are reluctant to ask for help because they believe asking implies an inability to live independently.
The most effective design strategy is to treat aesthetics from a functional point of view, where usability drives each detail, texture, size, and shape. These functional aesthetics provide visually rich cues communicating how to effectively engage with the product. Tactile and auditory feedback should also be leveraged to confirm successful usage.
Human factors engineering (HFE) breaks down the device usability problem by identifying the physical, cognitive, and emotional user requirements shaping and informing design. HFE characterizes how various diseases manifest and affect the hands in different ways. Defining these fundamental human factors are the key to creating designs that reinstate lost hand function and create a powerful, uplifting user experience that instills confidence and facilitates compliance. It is a profound error for device manufacturers to design a device then simply assign it to a disease state after the fact. A user-centered design approach that accounts for specific user populations is the only way to ensure these devices truly meet the needs of those suffering from chronic illness.
Dr. Bryce Rutter, founder and CEO of Metaphase Design Group Inc., is a leading expert in the research, ergonomics, and design of medical products, and a worldwide specialist in hand-intensive products and packaging. His work includes collaborations with numerous global prestigious brands and high-profile startups on products ranging from robotic surgical systems, powered and manual instrument design, and drug delivery systems to disposables, mobile and wearable devices, and personal care products to IFUs, as well as usability and contextual inquiry research programs. Metaphase delivers innovative designs that redefine industry standards, invigorate sleepy brands, and create new product categories. Under Dr. Rutter’s leadership, Metaphase has received more than 120 international design excellence awards and 117 patents. Dr. Rutter holds degrees in industrial design and a Ph.D. in kinesiology, specializing in hand function. Contact him at bryce@metaphase.com.
Device manufacturers today predominately use a design strategy that begins with engineering development of a device platform. R&D explores a wide variety of mechanical design options for calking and triggering a device, which in turn dictate its overall size, layout, and the forces required to reliably function. Only after the new device design is complete will manufacturers begin the search for a pharmaceutical partner that wants to use the device for its drug. It’s not surprising this engineering-driven design strategy leads to unintuitive and difficult to use device designs, because the device has been designed before it’s known which drug—and therefore, what type of user—will operate the device. This R&D strategy overlooks fundamental human factors considerations that directly impact usability. They have forgotten about the user.
It’s all about the user.
A user-centered design strategy is driven by how people think, feel, and behave. Mechanical design solutions meeting user needs can only be developed after truly understanding the user. Good device design begins with accommodating the widest possible range in hand, body size, and strength—ideally, the 5th percentile female to the 95th percentile male.
People commonly draw from one of four types of grips depending on the task’s complexity and the forces required to operate a device. When strength and endurance are required, people naturally gravitate toward power grips that recruit the larger forearm muscles. In contrast, for high precision and control, people default to hi-dexterity bilateral and trilateral grips that recruit smaller, weaker finger muscles.
All of these common grips change when disease steps in and alters how people can use their hands. For example, developing devices for people with rheumatoid arthritis (RA) presents a unique design challenge. The disease causes severe joint pain in the fingers and wrists and diminishes strength and dexterity. Living with these impairments forces users to routinely modify grasping and handling behaviors to compensate.
Grip touchpoints also change because RA users commonly experience skewed knuckle joints that laterally shifts where the fingertips land on the device. Furthermore, the disease dramatically reduces strength and range of joint motion, which erode grasping and dexterity. It is not uncommon to see a 60-80 percent reduction in finger strength and 50 percent reduction in the range of joint motion. These factors define how to design specialized grips that meet RA users’ unique needs.
Pain is also a significant design factor in designing for arthritic hands. To reduce pain, industrial designers and human factors engineers can identify device sizes and shapes that encourage power grips engaging fewer finger joints and recruiting the stronger forearm muscles.
Connected to hand function are considerations for how different anatomic injection sites influence usability. Line of sight, power versus precision grasping, trigger actuation forces, and the ability to stabilize the device throughout injection play key roles in creating easy-to-use and intuitive designs. The need to properly align the device to the skin surface to ensure proper needle penetration depth for drug delivery is equally important. Evaluating the ergonomics of these design requirements will define the most effective grasping strategy as it relates to the anatomic site and placing the device perpendicular to the injection site for successful drug delivery.
Design for Dignity
We often hear about “dying with dignity,” but rarely about living with dignity. When becoming ill, we experience a loss of dignity because we often lose autonomy and feel vulnerable. It is common for those suffering from a chronic illness to question their self-worth and become depressed. Poor mental health and disease-related physical impairments directly impact patient compliance.
The right look and feel of a device can make a powerful emotional connection with users. A plethora of ugly medical devices projecting an unpleasant stigma exist in today’s market—these practically advertise the user’s illness or disability. Fundamental functional design oversights requiring awkward grasping patterns and triggers or actuators not located under the natural fall of the fingertip and frequently exceed the user’s strength exacerbate this stigma.
A poorly designed, unintuitive, hard-to-use product impacts how people feel about themselves. Struggling with a device is both frustrating and embarrassing. Bad designs create feelings of defeat, and many users are reluctant to ask for help because they believe asking implies an inability to live independently.
The most effective design strategy is to treat aesthetics from a functional point of view, where usability drives each detail, texture, size, and shape. These functional aesthetics provide visually rich cues communicating how to effectively engage with the product. Tactile and auditory feedback should also be leveraged to confirm successful usage.
Human factors engineering (HFE) breaks down the device usability problem by identifying the physical, cognitive, and emotional user requirements shaping and informing design. HFE characterizes how various diseases manifest and affect the hands in different ways. Defining these fundamental human factors are the key to creating designs that reinstate lost hand function and create a powerful, uplifting user experience that instills confidence and facilitates compliance. It is a profound error for device manufacturers to design a device then simply assign it to a disease state after the fact. A user-centered design approach that accounts for specific user populations is the only way to ensure these devices truly meet the needs of those suffering from chronic illness.
Dr. Bryce Rutter, founder and CEO of Metaphase Design Group Inc., is a leading expert in the research, ergonomics, and design of medical products, and a worldwide specialist in hand-intensive products and packaging. His work includes collaborations with numerous global prestigious brands and high-profile startups on products ranging from robotic surgical systems, powered and manual instrument design, and drug delivery systems to disposables, mobile and wearable devices, and personal care products to IFUs, as well as usability and contextual inquiry research programs. Metaphase delivers innovative designs that redefine industry standards, invigorate sleepy brands, and create new product categories. Under Dr. Rutter’s leadership, Metaphase has received more than 120 international design excellence awards and 117 patents. Dr. Rutter holds degrees in industrial design and a Ph.D. in kinesiology, specializing in hand function. Contact him at bryce@metaphase.com.