Business Wire10.23.19
Phillips-Medisize, a Molex company, announced an agreement with a leading biotechnology company to manufacture a wearable, electronic-enabled combination product. The product is a post-phase III development-stage combination drug and device that combines two-day, single use, disposable technology for subcutaneous drug delivery.
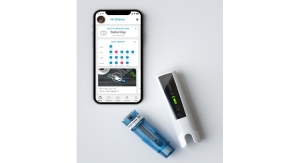
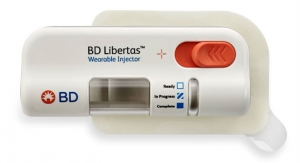
The wearable combination product—which is replaced every two days—is about the size of a hockey puck, and can either be adhered to the patient’s body, via an adhesive patch, or worn near the infusion site.
Phillips-Medisize, which built devices and supplied key data to support the FDA NDA submission, will provide final assembly, testing and quality control of this electronic combination product. Specifically, Phillips-Medisize will handle the prefilled drug cartridge that fits inside the device. This drug container is aseptically filled and shipped to Phillips-Medisize. Several state-of-the-art Phillips-Medisize facilities are involved in various stages of manufacturing the combination product—including molding, assembly, drug handling, labeling, reconciliation and more.
“This exciting new contract taps over 13 years of Phillips-Medisize’s combination product expertise, which includes cold chain drug handling and first programs with API handling. We have experience in manufacturing both combination and electronic-enabled drug delivery devices, and this is our first product submitted for FDA approval that includes both,” said Matt Jennings, CEO and president, Phillips-Medisize.
Pending receipt of FDA approval, Phillips-Medisize is contracted to provide commercialization services from its Hudson, WI-based manufacturing site. The combination product is slated for launch in 2H 2020. Backed by Phillips-Medisize’s scalability to move to high volume production, the product will be well positioned for a successful market launch.
Strong potential also exists to bring the device onto a connected health platform in the future, by embedding low-cost connectivity that will make it possible to capture and communicate data between patient and provider in order to help monitor adherence.
The wearable combination product—which is replaced every two days—is about the size of a hockey puck, and can either be adhered to the patient’s body, via an adhesive patch, or worn near the infusion site.
Phillips-Medisize, which built devices and supplied key data to support the FDA NDA submission, will provide final assembly, testing and quality control of this electronic combination product. Specifically, Phillips-Medisize will handle the prefilled drug cartridge that fits inside the device. This drug container is aseptically filled and shipped to Phillips-Medisize. Several state-of-the-art Phillips-Medisize facilities are involved in various stages of manufacturing the combination product—including molding, assembly, drug handling, labeling, reconciliation and more.
“This exciting new contract taps over 13 years of Phillips-Medisize’s combination product expertise, which includes cold chain drug handling and first programs with API handling. We have experience in manufacturing both combination and electronic-enabled drug delivery devices, and this is our first product submitted for FDA approval that includes both,” said Matt Jennings, CEO and president, Phillips-Medisize.
Pending receipt of FDA approval, Phillips-Medisize is contracted to provide commercialization services from its Hudson, WI-based manufacturing site. The combination product is slated for launch in 2H 2020. Backed by Phillips-Medisize’s scalability to move to high volume production, the product will be well positioned for a successful market launch.
Strong potential also exists to bring the device onto a connected health platform in the future, by embedding low-cost connectivity that will make it possible to capture and communicate data between patient and provider in order to help monitor adherence.