Britt Jones, Manager of Package Testing Services, WuXi AppTec06.13.19
With recent updates to ISO 11607, Part 1 and Part 2, and the Medical Device Regulation (MDR) on track to replace Europe’s current Medical Device Directive (MDD) in 2020, medical device manufacturers are working hard to ensure their package designs are in line with impending regulatory changes. In short, updates to ISO 11607, Part 1 and Part 2, introduce stricter sterile barrier requirements and techniques for introducing devices to the sterile field, thus potentially requiring time-consuming package redesigns and revalidations, which is why it is important to think about testing and validations now.
Ultimately, updates to ISO 11607 should help manufacturers comply with MDR, as the goal of the revisions is to harmonize the standards with the General Safety and Performance Requirements of the MDR. Packaging failure or the inability to demonstrate package integrity per the revised standards could have serious business implications. Noncompliance may result in a number of negative outcomes, such as having a product pulled from market, unforeseen costs associated with package redesign and/or retesting, or reputation damage if a package fails and compromises patient safety.
At the end of the day, MDR and ISO standards updates aim to ensure patient safety and improve care outcomes by establishing a baseline for preclinical device safety testing. There are three main objectives behind the ISO 11607, Part 1 and Part 2, revisions. The first focuses on harmonizing the terminology used throughout the documents to mirror other international standards; the second is the addition of a usability requirement; and the third outlines packaging inspection requirements. Let’s dive deeper into each of these objectives and how they will affect your products and preclinical device safety test plan.
Harmonization of Terminology
Terminology has been updated in ISO 11607, Part 1 and Part 2, to create clear and consistent expectations for validation testing in existing and emerging markets outside the United States. Standardizing the language also aims to better harmonize definitions with ISO 11139. While changes to the document’s vocabulary may appear as minor details, harmonizing terms helps establish a globalized set of standards and a mutual understanding of proper practices for packaging, labeling, and integrity testing.
Usability Evaluation—The Ability to Identify Where to Begin Opening
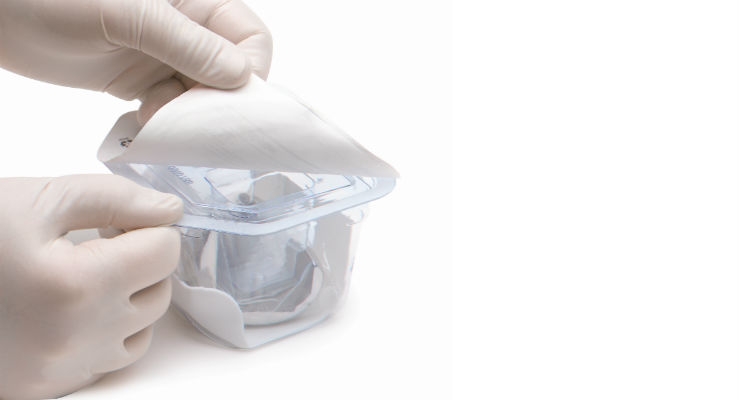
The second update adds a usability evaluation requirement. Usability is the healthcare professional use evaluation. With downstream impacts in mind, the changes made to ISO 11607 regarding usability are directly related to new MDR requirements. The updates to Part 1 specify what is necessary as far as “the final hundred yards,” or the point at which the medical device is being introduced to the sterile field. This change is prominent, as it requires that all packaging specify a clear location at which to begin opening the sterile barrier system in a way that prevents contaminating or damaging the contents. Providing clear instructions for performing the technique to open and present the product in the sterile field is particularly important for infection prevention. This update will also require OEMs to complete a usability study to demonstrate the instructions and packaging design are intuitive and allow introduction to the sterile field.
Sterile Barrier System—The Ability to Subsequently Present the Contents Aseptically
The third and final update presents new information regarding the inspection of sterile barrier systems prior to use. The objective behind this update is to ensure the package remains intact and able to protect the product inside from microbial contamination. From the moment the package is opened and taken into the sterile field, it must remain clear how to properly present the product, as well as what steps practitioners need to take to ensure safe and proper use.
Package Testing & Validation: What You Need to Know
Package testing is where remaining variance between ISO 11607 and MDR exists. Testing will be required to comply with ISO 11607. Determining which tests are needed involves a risk analysis under ISO 16775. Prompt revalidation is also required after any packaging changes, and reporting is required if there are any changes to materials or handling processes. For MDR, on the other hand, retesting to the latest standard is not required, but thorough justification is still required for compliance. In reality, many legacy products on the market require more evidence than is available, even after a gap analysis is performed. For these reasons, both MDR and ISO 11607, Part 1 and Part 2, require package integrity testing, in most cases.
Procedures required for package integrity testing will vary by device, but there are three main methods.
Validation Requirements
Package validation involves integrity testing of the sterile barrier system on a sterilized product after real-time aging, accelerated aging, and simulated distribution challenges are complete.
The package stability portion of the validation can be accomplished using accelerated-aging data until real-time data becomes available. Performance testing of the sterile barrier system is demonstrated through simulated distribution testing, which includes worst-case packaging configuration. Package integrity testing, such as seal peel strength, bubble emission, etc., is performed after stability and performance testing to demonstrate the sterile barrier system adequately protects the device from microbial contamination.
Selecting the Right Partner
Outsourcing your package testing and validation needs to an experienced contract research organization (CRO) can help ensure your Is are dotted and Ts are crossed, saving you a headache. The following questions are thought-starters to help make the partnership a success.
Questions you should ask:
Questions you should be prepared to answer:
Conclusion
Package validations are a key component of all medical device design history files and regulatory submissions. While ensuring all devices are compliant with updated standards by the May 26, 2020 MDR deadline may seem daunting, you don’t have to face the process alone. Doing internal due diligence and prioritizing partner selection can mitigate risk for lost time and wasted resources. While there is no formulaic process to promise a quick and painless path to regulatory approval, these tips will get you on the right track. v
Note: This column is part one of a two-part series that examines the impact of the MDR on medical device manufacturers. While this part is focused on packaging, the second part will highlight more of the broad effect of the new EU regulations.
Britt Jones is the manager of package testing services for devices, biologics, and combination products at WuXi AppTec. He serves on the ASTM Committee on Packaging (ASTM D-10) and is a member of and certified by the International Safe Transit Association (ISTA) and the Institute of Packaging Professionals (IoPP). He can be reached at britt.jones@wuxiapptec.com.
Ultimately, updates to ISO 11607 should help manufacturers comply with MDR, as the goal of the revisions is to harmonize the standards with the General Safety and Performance Requirements of the MDR. Packaging failure or the inability to demonstrate package integrity per the revised standards could have serious business implications. Noncompliance may result in a number of negative outcomes, such as having a product pulled from market, unforeseen costs associated with package redesign and/or retesting, or reputation damage if a package fails and compromises patient safety.
At the end of the day, MDR and ISO standards updates aim to ensure patient safety and improve care outcomes by establishing a baseline for preclinical device safety testing. There are three main objectives behind the ISO 11607, Part 1 and Part 2, revisions. The first focuses on harmonizing the terminology used throughout the documents to mirror other international standards; the second is the addition of a usability requirement; and the third outlines packaging inspection requirements. Let’s dive deeper into each of these objectives and how they will affect your products and preclinical device safety test plan.
Harmonization of Terminology
Terminology has been updated in ISO 11607, Part 1 and Part 2, to create clear and consistent expectations for validation testing in existing and emerging markets outside the United States. Standardizing the language also aims to better harmonize definitions with ISO 11139. While changes to the document’s vocabulary may appear as minor details, harmonizing terms helps establish a globalized set of standards and a mutual understanding of proper practices for packaging, labeling, and integrity testing.
Usability Evaluation—The Ability to Identify Where to Begin Opening
The second update adds a usability evaluation requirement. Usability is the healthcare professional use evaluation. With downstream impacts in mind, the changes made to ISO 11607 regarding usability are directly related to new MDR requirements. The updates to Part 1 specify what is necessary as far as “the final hundred yards,” or the point at which the medical device is being introduced to the sterile field. This change is prominent, as it requires that all packaging specify a clear location at which to begin opening the sterile barrier system in a way that prevents contaminating or damaging the contents. Providing clear instructions for performing the technique to open and present the product in the sterile field is particularly important for infection prevention. This update will also require OEMs to complete a usability study to demonstrate the instructions and packaging design are intuitive and allow introduction to the sterile field.
Sterile Barrier System—The Ability to Subsequently Present the Contents Aseptically
The third and final update presents new information regarding the inspection of sterile barrier systems prior to use. The objective behind this update is to ensure the package remains intact and able to protect the product inside from microbial contamination. From the moment the package is opened and taken into the sterile field, it must remain clear how to properly present the product, as well as what steps practitioners need to take to ensure safe and proper use.
Package Testing & Validation: What You Need to Know
Package testing is where remaining variance between ISO 11607 and MDR exists. Testing will be required to comply with ISO 11607. Determining which tests are needed involves a risk analysis under ISO 16775. Prompt revalidation is also required after any packaging changes, and reporting is required if there are any changes to materials or handling processes. For MDR, on the other hand, retesting to the latest standard is not required, but thorough justification is still required for compliance. In reality, many legacy products on the market require more evidence than is available, even after a gap analysis is performed. For these reasons, both MDR and ISO 11607, Part 1 and Part 2, require package integrity testing, in most cases.
Procedures required for package integrity testing will vary by device, but there are three main methods.
- Package integrity and seal strength testing: Package integrity and seal strength limitations must be established to qualify package systems for post-sterilization production, shipping, and shelf life. Physical testing, such as bubble emission, dye penetration, seal strength, and others, can often provide greater sensitivity than microbial challenge tests and are oftentimes the preferred methods used in package validations.
- Accelerated aging and shelf-life stability: Aging tests are essential in determining shelf-life limitations and creating expiration dating used on product packaging. Understanding accelerated-aging data can help answer questions until real-time aging data becomes available. Aging additional samples may be useful in the event that an unforeseen issue arises. Generally, if an issue comes up, a manufacturer would pull retains and explore what the real-time product data tells. Retains would be comparable to the product in the field—generally a sampling of the commercial lot. If necessary, a device manufacturer may also condition the samples to explore whether transport or storage conditions factored into the field issue.
- Simulated distribution testing: Due to the possibility of a package becoming damaged (e.g., puncture, abrasion, or seal failure during distribution), manufacturers must sufficiently vet the ability of the package and shipper to protect the product through handling, shipping, and other distribution processes.
Validation Requirements
Package validation involves integrity testing of the sterile barrier system on a sterilized product after real-time aging, accelerated aging, and simulated distribution challenges are complete.
The package stability portion of the validation can be accomplished using accelerated-aging data until real-time data becomes available. Performance testing of the sterile barrier system is demonstrated through simulated distribution testing, which includes worst-case packaging configuration. Package integrity testing, such as seal peel strength, bubble emission, etc., is performed after stability and performance testing to demonstrate the sterile barrier system adequately protects the device from microbial contamination.
Selecting the Right Partner
Outsourcing your package testing and validation needs to an experienced contract research organization (CRO) can help ensure your Is are dotted and Ts are crossed, saving you a headache. The following questions are thought-starters to help make the partnership a success.
Questions you should ask:
- What are your accelerated aging capabilities? CROs should be able to perform testing in a variety of settings. For reliable aging results, your CRO’s capabilities should allow it to perform accelerated aging at multiple parameters to ensure all package types and materials are compatible with the temperature being used. Accelerated-aging data can be used to establish shelf life until real-time data is available.
- Is your distribution/transportation testing performed in-house? It is important that your CRO performs this testing in-house to avoid additional handling and hazards associated with the distribution environment. If a CRO outsources this function, it can increase risk for unreliable data.
- Do you have the ability to do environmental conditioning? CROs should be able to perform environmental conditioning testing, which exposes packages to freezing temperatures, as well as tropical wet and dry climates for impact assessment. This data helps support a package system’s ability to withstand various “worst-case” scenarios.
Questions you should be prepared to answer:
- What is your package type? It may seem obvious, but preparing information that specifies your package material and dimensions will allow you to move through the validation process more quickly.
- What type of product will be housed inside the package? While packaging makes up the sterile barrier system, it also provides a measure of device protection. CROs need to know the level of product fragility in these cases to determine how much protection is necessary.
- Is there any preexisting data that helps support the safety of the device? For example, sometimes accelerated-aging data can help CROs determine the targeted shelf life and offer justification for the length of time a sample is aged.
- How is your device packaged? For example, is it a single or a multi-pack? Is there a shelf carton? Understanding your approach to packaging will help CROs provide more specific recommendations for your needs.
- What is your organization’s end goal? To ensure efficient and effective testing, you must communicate the goal for distribution—full package validation or partial validation?
Conclusion
Package validations are a key component of all medical device design history files and regulatory submissions. While ensuring all devices are compliant with updated standards by the May 26, 2020 MDR deadline may seem daunting, you don’t have to face the process alone. Doing internal due diligence and prioritizing partner selection can mitigate risk for lost time and wasted resources. While there is no formulaic process to promise a quick and painless path to regulatory approval, these tips will get you on the right track. v
Note: This column is part one of a two-part series that examines the impact of the MDR on medical device manufacturers. While this part is focused on packaging, the second part will highlight more of the broad effect of the new EU regulations.
Britt Jones is the manager of package testing services for devices, biologics, and combination products at WuXi AppTec. He serves on the ASTM Committee on Packaging (ASTM D-10) and is a member of and certified by the International Safe Transit Association (ISTA) and the Institute of Packaging Professionals (IoPP). He can be reached at britt.jones@wuxiapptec.com.