Florence Joffroy-Black and Dave Sheppard, MedWorld Advisors04.03.19
There is no denying the innovative and disruptive nature of the medtech industry, or its constant evolution. Success in this space has long depended largely on flexibility and a commitment to change, as reformation can help companies avoid obsolescence and extinction and enable them to capitalize on the sector’s projected 4.5 percent compound annual growth rate through 2023. It is important for medical device firms to continuously address key issues to ensure their business is moving forward. Worldwide industry dynamics and technology trends are creating both opportunity and confusion in the international marketplace.
The forecasted growth in Figure 1 represents a tremendous opportunity for all medtech companies. To take full advantage of this opportunity, however, organizations must have transparent leadership and a solid change management plan that clearly outlines industry challenges and prospects, and encourages executives to create and prioritize growth strategies.
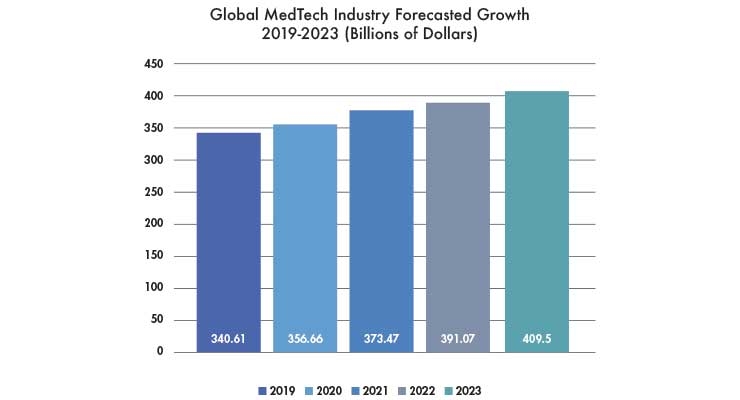
Figure 1: Source - MedWorld Advisors Market Research
There are various factors to consider in drafting such a plan. In the United States, companies should prepare themselves for a possible return of the 2.3 percent medical device tax (if it isn’t suspended or repealed this year), and understand that clinical outcomes are now key to reimbursement.
To minimize the chances of the device tax resurfacing, companies might consider lobbying their Congressional representatives to remind them of the levy’s detrimental effect on medical technology, and undertake “scenario planning” based on the tax’s 2020 return.
Regardless of the device tax’s fate next year, though, companies must understand that growth is no longer being driven by incremental innovation but rather by reimbursement and clinical evidence. Reimbursement is contingent on equivalent efficacy (necessary for U.S. Food and Drug Administration approval) and cost savings. Medtech firms must challenge themselves to prove their economic value to customers.
Growth in Europe, on the other hand, will be driven mostly by changes in the Medical Device Regulation (MDR) and country-specific marketing strategies.
For years, companies launching medical products debuted them first in Europe due to a less complex regulatory process. But the new MDR—set to take effect next year—will reverse that order; companies, therefore, would be wise to address any shortcomings in their regulatory strategies that could prevent them from complying with the new standards.
Organizations must also adjust their marketing approach in Europe. Rather than target products to the continent as a whole, companies must tailor their innovations by country; such a shift requires geographically differentiated and prioritized marketing plans.
Asia’s Divergent Dynamics
Conflicting forces at work in Asia require country-specific marketing and growth strategies as well. Japan, for example, is contending with changing demographics, shrinking hospital budgets, and skyrocketing home health expenses. These elements are driving a need for lower-cost solutions such as those afforded in both the home health and nursing care settings.
China, contrarily, has experienced explosive growth in its medtech sector (though it’s slowed somewhat lately). Complicating this opportunity, however, is the decentralization of reimbursement decisions and tariffs at city, county, and province levels, which often result in fragmented and complex coverage policies. Adding to the Middle Kingdom’s complexities is the medical device authority’s name change (from China Food and Drug Administration to National Medical Products Administration), effective Sept. 1, 2018. Any foreign company registering medical devices in China must now use the new name and abbreviation.
Navigating China’s regulatory system and distribution channels can be ameliorated with the proper local structure and the help of a good partner. Having the right local structure may include establishing a local manufacturing presence (production “in-country for in-country sales” strategy) or a sales team to manage distributors (note: even local China manufacturers use distributors at the hospital level). Companies that choose an outside “in-China” partner to manage their sales and distribution channels should maintain oversight to ensure their collaborators provide enough attention to their businesses.
With the size of its Tier 1 cities (equivalent to roughly 50 percent of the U.S. market), India clearly represents one of the best growth opportunities in Asia. While its regulatory process is not nearly as burdensome as other countries, its infrastructure, distribution, demand for low-cost products, and continuously changing political landscape can make it a frustrating country in which to conduct business.
Education, however, is the key to success in India. Customers there are no different than anywhere else on the planet—they are looking for great products at the right value.
Latin America’s Dissipating Volatility
Although pockets of unpredictability remain (i.e., Venezuela), the majority of Latin American medtech markets are relatively stable. This region is often overlooked as a market. But when targeting the right countries, this market can sometimes represent one of the easier paths to international expansion, especially for U.S. companies.
MENA: A Springboard for Global Expansion
The Middle East/Africa region may seem like a small and inconsequential player on the global stage, but it’s a great entry point for companies looking to rapidly expand internationally. Africa (outside of South Africa, actually) can sometimes be difficult for foreign companies to navigate but a bit of research and focused targeting will usually yield satisfying results, as many of the key Middle East countries are hungry for good technology.
All of these global issues and opportunities have direct impact on companies selling medical products in the global marketplace, regardless of whether they are an OEM or a supplier. Remember: the best suppliers are those that help their customers succeed and grow.
This is a worldwide marketplace, and medical device firms must be prepared for the opportunities available in the various regions. Most importantly, they must prepare their management teams to create and execute the right actions/plans to succeed in the current and future global market.
Florence Joffroy-Black, CM&AA, is a longtime marketing and M&A expert with significant experience in the medical technology industry, including working for multi-national corporations based in the United States, Germany, and Israel. She can be reached at florencejblack@medworldadvisors.com.
Dave Sheppard, CM&AA, is a former medical technology Fortune 500 executive and is now a managing director at MedWorld Advisors. He can be reached at davesheppard@medworldadvisors.com.
The forecasted growth in Figure 1 represents a tremendous opportunity for all medtech companies. To take full advantage of this opportunity, however, organizations must have transparent leadership and a solid change management plan that clearly outlines industry challenges and prospects, and encourages executives to create and prioritize growth strategies.

Figure 1: Source - MedWorld Advisors Market Research
There are various factors to consider in drafting such a plan. In the United States, companies should prepare themselves for a possible return of the 2.3 percent medical device tax (if it isn’t suspended or repealed this year), and understand that clinical outcomes are now key to reimbursement.
To minimize the chances of the device tax resurfacing, companies might consider lobbying their Congressional representatives to remind them of the levy’s detrimental effect on medical technology, and undertake “scenario planning” based on the tax’s 2020 return.
Regardless of the device tax’s fate next year, though, companies must understand that growth is no longer being driven by incremental innovation but rather by reimbursement and clinical evidence. Reimbursement is contingent on equivalent efficacy (necessary for U.S. Food and Drug Administration approval) and cost savings. Medtech firms must challenge themselves to prove their economic value to customers.
Growth in Europe, on the other hand, will be driven mostly by changes in the Medical Device Regulation (MDR) and country-specific marketing strategies.
For years, companies launching medical products debuted them first in Europe due to a less complex regulatory process. But the new MDR—set to take effect next year—will reverse that order; companies, therefore, would be wise to address any shortcomings in their regulatory strategies that could prevent them from complying with the new standards.
Organizations must also adjust their marketing approach in Europe. Rather than target products to the continent as a whole, companies must tailor their innovations by country; such a shift requires geographically differentiated and prioritized marketing plans.
Asia’s Divergent Dynamics
Conflicting forces at work in Asia require country-specific marketing and growth strategies as well. Japan, for example, is contending with changing demographics, shrinking hospital budgets, and skyrocketing home health expenses. These elements are driving a need for lower-cost solutions such as those afforded in both the home health and nursing care settings.
China, contrarily, has experienced explosive growth in its medtech sector (though it’s slowed somewhat lately). Complicating this opportunity, however, is the decentralization of reimbursement decisions and tariffs at city, county, and province levels, which often result in fragmented and complex coverage policies. Adding to the Middle Kingdom’s complexities is the medical device authority’s name change (from China Food and Drug Administration to National Medical Products Administration), effective Sept. 1, 2018. Any foreign company registering medical devices in China must now use the new name and abbreviation.
Navigating China’s regulatory system and distribution channels can be ameliorated with the proper local structure and the help of a good partner. Having the right local structure may include establishing a local manufacturing presence (production “in-country for in-country sales” strategy) or a sales team to manage distributors (note: even local China manufacturers use distributors at the hospital level). Companies that choose an outside “in-China” partner to manage their sales and distribution channels should maintain oversight to ensure their collaborators provide enough attention to their businesses.
With the size of its Tier 1 cities (equivalent to roughly 50 percent of the U.S. market), India clearly represents one of the best growth opportunities in Asia. While its regulatory process is not nearly as burdensome as other countries, its infrastructure, distribution, demand for low-cost products, and continuously changing political landscape can make it a frustrating country in which to conduct business.
Education, however, is the key to success in India. Customers there are no different than anywhere else on the planet—they are looking for great products at the right value.
Latin America’s Dissipating Volatility
Although pockets of unpredictability remain (i.e., Venezuela), the majority of Latin American medtech markets are relatively stable. This region is often overlooked as a market. But when targeting the right countries, this market can sometimes represent one of the easier paths to international expansion, especially for U.S. companies.
MENA: A Springboard for Global Expansion
The Middle East/Africa region may seem like a small and inconsequential player on the global stage, but it’s a great entry point for companies looking to rapidly expand internationally. Africa (outside of South Africa, actually) can sometimes be difficult for foreign companies to navigate but a bit of research and focused targeting will usually yield satisfying results, as many of the key Middle East countries are hungry for good technology.
All of these global issues and opportunities have direct impact on companies selling medical products in the global marketplace, regardless of whether they are an OEM or a supplier. Remember: the best suppliers are those that help their customers succeed and grow.
This is a worldwide marketplace, and medical device firms must be prepared for the opportunities available in the various regions. Most importantly, they must prepare their management teams to create and execute the right actions/plans to succeed in the current and future global market.
Florence Joffroy-Black, CM&AA, is a longtime marketing and M&A expert with significant experience in the medical technology industry, including working for multi-national corporations based in the United States, Germany, and Israel. She can be reached at florencejblack@medworldadvisors.com.
Dave Sheppard, CM&AA, is a former medical technology Fortune 500 executive and is now a managing director at MedWorld Advisors. He can be reached at davesheppard@medworldadvisors.com.

























