Daniel R. Matlis, President, Axendia Inc.06.13.19
Note: This is the second in a two-part series. Read the first part by clicking here.
Supply chain dynamics are prompting life science companies to seek innovative approaches that improve product safety while also enhancing clinical outcomes, reducing costs and risks, and ensuring regulatory compliance. To attain the sustained benefits of globalization, the life science products ecosystem must implement a new paradigm to better manage global supply chains.
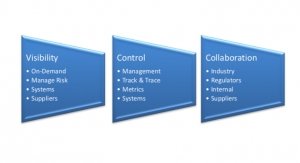
Companies can no longer rely on traditional supplier management methods; they must implement new strategies that help to reduce and control risks. This calls for changing the business, technology, and regulatory models traditionally used in the industry. The three key components to managing this shift are on-demand visibility, supply network control, and collaboration.
Control Over the Global Supply Network
Traditionally, life science product manufacturers relied on testing quality out of the process to ensure only the highest quality products reached consumers.
The thrust of the Quality Metrics initiatives focuses on gaining deep process and product understanding to ensure tight controls over critical-to-quality parameters. As a result, the product emerging from these highly controlled processes would meet quality standards without the need for testing at the completion of each manufacturing operation.
Today, supply chain control extends well past the ingredients and components passing though brand owners’ receiving docks. Companies must maintain control of their products once they leave their facilities. Counterfeiting, product diversion, and thefts are on the rise, and brand owners must deploy systems and technologies to provide visibility throughout the entire value chain.
These new paradigms enable forward-thinking life science organizations to implement advanced control strategies to speed innovation, improve quality, and lower costs while facilitating regulatory compliance.
Supplier Network Control Roadmap
Life science companies must gain tighter control over the complete supply and value chains, from ingredient to patient. To attain this goal, brand owners should consider shifting from supply chains to supplier networks.
Supply chain control and collaboration should not invoke a “do it my way” mentality from brand owners, but rather benefit all constituents in the life science value chain.
Supply chains usually provide for only two touch points at each end of every link; yet, contemporary global supply, distribution, and repackaging arrangements require more than two points. It pretty much demands the availability of on-demand visibility at every step of the process.
In addition, brand owners should consider implementing risk-based supplier management strategies. Not every component/supplier has the same value and risk profile, but everyone must be in place to complete the product. Companies should assess suppliers based on their total value (including risk profiles), not just commodity costs.
Brand owners should also consider conducting on-demand remote supplier audits to ensure quality systems are followed at all times. Moreover, they should also employ “trust but verify” strategies to ensure their partners’ adherence to quality practices, processes, and procedures.
Additionally, manufacturers should implement approaches to access on-demand visibility across their supply networks. Such a strategy enables companies to shift from reactive to predictive problem-solving and root cause analysis.
Implementing technological, commercial, and legal frameworks promotes an on-demand information exchange that helps supplier and value networks become an extension of a brand owner’s own facility using standards-based and interoperable data.
Adequate control often requires deep process and product understanding. Thus, companies should encourage supplier collaboration so contractors better understand key quality parameters and implement systems that provide visibility to support internal process control based on actual product attributes, rather than incoming inspection testing.
To achieve control externally, brand owners should deploy processes and systems that enable them to connect the dots and bridge the gaps, regardless of the manufacturer or location of a component. To this end, partners and suppliers should be considered extensions of the company, providing components to the whole organization; they should not function as disconnected manufacturing islands providing unrelated parts.
Brand owners should integrate rigid command and control systems (like ERP and QMS) with cloud-based supply chain intelligence infrastructures. This would enable companies to realize the cost and agility benefits of global supply networks while ensuring product quality and safeguarding brand reputation.
To achieve continued supply network control and visibility, companies must reduce opportunities for counterfeiting, diversion, and theft. To this end, all life science product stakeholders should work cooperatively and diligently to implement standards to support the rollout of track and trace systems.
These approaches will enable companies and suppliers to close the loop across the supply chain by providing visibility, control, and actionable intelligence, regardless of supply and manufacturing strategies (in-house and outsourced).
Collaboration
When much of a company’s business operations existed within four walls, the need to collaborate with external partners was somewhat limited. But in today’s global environment, isolation makes it difficult to succeed. Regulators recognize this changing environment and are consequently embracing partnership and harmonization initiatives with industry and other life science stakeholders.
Collaboration also means changing the ways companies are structured to support the greater demand for cross-functional skills supporting global supply chains. It is no longer just a purchasing exercise to save costs. Global supply networks require a well-balanced team approach to minimize risk, maximize value, and ensure product safety.
It also means changing the way companies deal with suppliers; both sides should regard the relationship as a collaborative one where the interchange begins sooner in the process. In addition, it means engaging suppliers willing to collect and share information because they understand such a practice is in the best interest of all stakeholders.
Collaboration Roadmap
The word partnership has sometimes been used to describe the regulator-industry relationship that sustains product safety and effectiveness, but this alliance has often been an uneven one. More often than not, regulators told companies what to do and brand owners generally complied. While that is still the case, making this partnership work in a global environment will not be possible without collaboration between both sides to shape desired practices. These practices must embody reasonable approaches, which can be broadly leveraged by both industry and regulators. The scope of this global reality for individual companies or agencies will be impossible to achieve given the financial limitations in place.
Because of the size and complexity of global supply networks, the most viable and cost effective path to achieve on-demand visibility across the life science ecosystem involves sharing information to ensure product safety and efficacy. Information sharing can:
Cross-functional teams are an integral part of today’s global supply networks. Interactions between companies and suppliers are shifting from casual exchanges to long-term, stable relationships. Global supply networks demand a new collaborative approach to assess supplier risk, and these networks act proactively to address trends before they become non-conformances.
Expectations and requirements of these relationships should be made clear to every member of the supply network, especially critical partners. Supplier participation is a critical part of creating reasonable working relationships to meet requirements. To establish an honest dialogue, companies and their partners must clearly see the value and impact of the relationship. Suppliers must challenge assumptions and work together with brand owners toward improved solutions; they should not positively respond to every demand unless they can consistently deliver on those mandates.
There are many benefits of collaboration and joint planning between companies and suppliers. Working together for continuous improvement can drive lower costs through improved yields, fewer returns, and less rework. These lower costs can give the supplier greater flexibility in maintaining profitability while continuing to be a competitive resource for companies.
Conclusion
Supply chain dynamics are driving the life science ecosystem to seek innovative approaches that improve product safety, enhance clinical outcomes, reduce costs and risks, and ensure regulatory compliance. To attain the sustained benefits of globalization, industry must implement a new paradigm to manage global supply chains.
Brand owners must implement global supply chain strategies that capitalize on the benefits of globalization and outsourcing while proactively reducing and controlling risks. This calls for changing traditional business, technology, and regulatory models. To support these initiatives, industry must implement on-demand visibility, supplier control, and collaboration.
Daniel R. Matlis is founder and president of Axendia, an analyst firm providing advice on business, technology, and regulatory matters. He has almost three decades of experience spanning the health-science value chain. He has been an active member in FDA’s Case for Quality Initiative since 2014 and has presented Axendia’s research findings to industry executives and the FDA officials. Matlis began his career at Johnson & Johnson, where he provided leadership in technology, regulatory compliance and business. Before founding Axendia, Matlis was a partner, vice president, and general manager at a leading life science consultancy firm.
Supply chain dynamics are prompting life science companies to seek innovative approaches that improve product safety while also enhancing clinical outcomes, reducing costs and risks, and ensuring regulatory compliance. To attain the sustained benefits of globalization, the life science products ecosystem must implement a new paradigm to better manage global supply chains.
Companies can no longer rely on traditional supplier management methods; they must implement new strategies that help to reduce and control risks. This calls for changing the business, technology, and regulatory models traditionally used in the industry. The three key components to managing this shift are on-demand visibility, supply network control, and collaboration.
Control Over the Global Supply Network
Traditionally, life science product manufacturers relied on testing quality out of the process to ensure only the highest quality products reached consumers.
The thrust of the Quality Metrics initiatives focuses on gaining deep process and product understanding to ensure tight controls over critical-to-quality parameters. As a result, the product emerging from these highly controlled processes would meet quality standards without the need for testing at the completion of each manufacturing operation.
Today, supply chain control extends well past the ingredients and components passing though brand owners’ receiving docks. Companies must maintain control of their products once they leave their facilities. Counterfeiting, product diversion, and thefts are on the rise, and brand owners must deploy systems and technologies to provide visibility throughout the entire value chain.
These new paradigms enable forward-thinking life science organizations to implement advanced control strategies to speed innovation, improve quality, and lower costs while facilitating regulatory compliance.
Supplier Network Control Roadmap
Life science companies must gain tighter control over the complete supply and value chains, from ingredient to patient. To attain this goal, brand owners should consider shifting from supply chains to supplier networks.
Supply chain control and collaboration should not invoke a “do it my way” mentality from brand owners, but rather benefit all constituents in the life science value chain.
Supply chains usually provide for only two touch points at each end of every link; yet, contemporary global supply, distribution, and repackaging arrangements require more than two points. It pretty much demands the availability of on-demand visibility at every step of the process.
In addition, brand owners should consider implementing risk-based supplier management strategies. Not every component/supplier has the same value and risk profile, but everyone must be in place to complete the product. Companies should assess suppliers based on their total value (including risk profiles), not just commodity costs.
Brand owners should also consider conducting on-demand remote supplier audits to ensure quality systems are followed at all times. Moreover, they should also employ “trust but verify” strategies to ensure their partners’ adherence to quality practices, processes, and procedures.
Additionally, manufacturers should implement approaches to access on-demand visibility across their supply networks. Such a strategy enables companies to shift from reactive to predictive problem-solving and root cause analysis.
Implementing technological, commercial, and legal frameworks promotes an on-demand information exchange that helps supplier and value networks become an extension of a brand owner’s own facility using standards-based and interoperable data.
Adequate control often requires deep process and product understanding. Thus, companies should encourage supplier collaboration so contractors better understand key quality parameters and implement systems that provide visibility to support internal process control based on actual product attributes, rather than incoming inspection testing.
To achieve control externally, brand owners should deploy processes and systems that enable them to connect the dots and bridge the gaps, regardless of the manufacturer or location of a component. To this end, partners and suppliers should be considered extensions of the company, providing components to the whole organization; they should not function as disconnected manufacturing islands providing unrelated parts.
Brand owners should integrate rigid command and control systems (like ERP and QMS) with cloud-based supply chain intelligence infrastructures. This would enable companies to realize the cost and agility benefits of global supply networks while ensuring product quality and safeguarding brand reputation.
To achieve continued supply network control and visibility, companies must reduce opportunities for counterfeiting, diversion, and theft. To this end, all life science product stakeholders should work cooperatively and diligently to implement standards to support the rollout of track and trace systems.
These approaches will enable companies and suppliers to close the loop across the supply chain by providing visibility, control, and actionable intelligence, regardless of supply and manufacturing strategies (in-house and outsourced).
Collaboration
When much of a company’s business operations existed within four walls, the need to collaborate with external partners was somewhat limited. But in today’s global environment, isolation makes it difficult to succeed. Regulators recognize this changing environment and are consequently embracing partnership and harmonization initiatives with industry and other life science stakeholders.
Collaboration also means changing the ways companies are structured to support the greater demand for cross-functional skills supporting global supply chains. It is no longer just a purchasing exercise to save costs. Global supply networks require a well-balanced team approach to minimize risk, maximize value, and ensure product safety.
It also means changing the way companies deal with suppliers; both sides should regard the relationship as a collaborative one where the interchange begins sooner in the process. In addition, it means engaging suppliers willing to collect and share information because they understand such a practice is in the best interest of all stakeholders.
Collaboration Roadmap
The word partnership has sometimes been used to describe the regulator-industry relationship that sustains product safety and effectiveness, but this alliance has often been an uneven one. More often than not, regulators told companies what to do and brand owners generally complied. While that is still the case, making this partnership work in a global environment will not be possible without collaboration between both sides to shape desired practices. These practices must embody reasonable approaches, which can be broadly leveraged by both industry and regulators. The scope of this global reality for individual companies or agencies will be impossible to achieve given the financial limitations in place.
Because of the size and complexity of global supply networks, the most viable and cost effective path to achieve on-demand visibility across the life science ecosystem involves sharing information to ensure product safety and efficacy. Information sharing can:
- Reduce redundant, costly audits for both industry and those being audited;
- Create a transparency of quality-related information to ensure product safety; and
- Improve performance.
Cross-functional teams are an integral part of today’s global supply networks. Interactions between companies and suppliers are shifting from casual exchanges to long-term, stable relationships. Global supply networks demand a new collaborative approach to assess supplier risk, and these networks act proactively to address trends before they become non-conformances.
Expectations and requirements of these relationships should be made clear to every member of the supply network, especially critical partners. Supplier participation is a critical part of creating reasonable working relationships to meet requirements. To establish an honest dialogue, companies and their partners must clearly see the value and impact of the relationship. Suppliers must challenge assumptions and work together with brand owners toward improved solutions; they should not positively respond to every demand unless they can consistently deliver on those mandates.
There are many benefits of collaboration and joint planning between companies and suppliers. Working together for continuous improvement can drive lower costs through improved yields, fewer returns, and less rework. These lower costs can give the supplier greater flexibility in maintaining profitability while continuing to be a competitive resource for companies.
Conclusion
Supply chain dynamics are driving the life science ecosystem to seek innovative approaches that improve product safety, enhance clinical outcomes, reduce costs and risks, and ensure regulatory compliance. To attain the sustained benefits of globalization, industry must implement a new paradigm to manage global supply chains.
Brand owners must implement global supply chain strategies that capitalize on the benefits of globalization and outsourcing while proactively reducing and controlling risks. This calls for changing traditional business, technology, and regulatory models. To support these initiatives, industry must implement on-demand visibility, supplier control, and collaboration.
Daniel R. Matlis is founder and president of Axendia, an analyst firm providing advice on business, technology, and regulatory matters. He has almost three decades of experience spanning the health-science value chain. He has been an active member in FDA’s Case for Quality Initiative since 2014 and has presented Axendia’s research findings to industry executives and the FDA officials. Matlis began his career at Johnson & Johnson, where he provided leadership in technology, regulatory compliance and business. Before founding Axendia, Matlis was a partner, vice president, and general manager at a leading life science consultancy firm.