Ranica Arrowsmith, Associate Editor09.09.15
Why do cell phones still suck?
No, really. If applying for a work-from-home position, most companies will insist you have a land line. Without one, telecommunications are unreliable; and even if your cell phone service carrier is the most reliable in the world, the basic sound quality still leaves a lot to be desired.
In a 2014 article cheekily titled “All Smart, No Phone,” Spectrum explained that cell phone carriers face several obstacles to achieving better voice quality standards. The first is simply an aging public switched telephone network. The public switched telephone network (PSTN) is the world’s collection of interconnected voice-oriented public telephone networks, both commercial and government-owned. It’s also referred to as the plain old telephone service (POTS). The second is hardware design. The original Motorola “brick” phones, one time a status symbol and now, a mere 30 years later, a joke, were constructed with voice calls in mind. They had large speakers about the size of an ear, and the microphone pointed directly towards the mouth. Which is easier to do with a mobile phone the size of an actual brick as opposed to one the size of a business card.
Chris Kyriakakis, founder and chief technology officer of Audyssey, a Los Angeles, Calif.–based acoustic design company, said the modern smartphone’s form “is driven by industrial design and not voice quality.” The screens are flat to facilitate scrolling, paging, thumbing, and all kinds of visual-targeted applications. The phone’s body is shaped and sized to fit as easily into the pocket as it does in the hand. This leaves little room for speaker components, which are compressed, dampening frequencies, making the richest of voices sound tinny and thin. Smartphone designers then add software to lessen such distortions. The microphone poses even more problems. For one, it doesn’t even face the mouth anymore. And one doesn’t need a degree in audiology to know that the further away a mic is from the mouth, the more background noise it picks up. Higher-end smartphones try to address this problem by using multiple microphones. The one closest to the mouth focus on the voice, while the ones further away compare sounds to the first mic to identify and then filter out unwanted sounds.
But in his seminal (and surrealist) text “The Little Prince,” Antoine de Saint Exupéry said, “Perfection is achieved not when there is nothing more to add, but when there is nothing left to take away.” Shaving down the smartphone to a more minimal, small, portable, wearable, pocketable, mobile form, and then adding features to it to make up for what was lost, can start to seem a little surreal in itself. This is not to pooh-pooh technological advancement. It is indeed quite a feat of modern science that we can carry around a device that allows us to communicate in voice and text form, watch video, listen to music, play games, manage our schedules and monitor our health in our pockets. But there must be a critical point at which miniaturization and technological add-ons converge and cease to advance.
Right?
The Golden Balance
Getting medical device components down to the “nano” (nanometer, or nm) level is pretty hip right now. At least, the term gets thrown around a lot. Just in July, Mequon, Wis.-based spine implant surface technology company Titan Spine hired a specialist to address precisely this problem: namely, that the notion of nanotechnology is not fully understood by the medical device industry. “One of Jim [Sevey’s] initial tasks will be to clearly differentiate the science of our nanotechnology platform from those that claim to have nanotechnology but have not been cleared by the FDA to do so,” Titan Spine President Kevin Gemas said of the company’s new hire.
But Gerry Murak is past nano. The CEO of Buffalo, N.Y.-based electronic contract manufacturer of printed circuit board assemblies, cables, wiring harness and electro-mechanical assemblies SoPark Corporation, which is ISO 13485 certified, has entered into a new venture with the State University of New York at Buffalo. Three researchers at the school have invented a disruptive technology that studies materials at the atomic level. Murak founded Precision Scientific Instruments Inc. (PSI) to license the technology that is touted to be able to examine materials at 50 times greater resolution than any other technology on the market.
“Sub-nano devices are the new frontier of state-of-the-art nano technology,” Murak claimed.
The PSi1 system, as it’s called, is hoped to allow researchers to unlock the commercial potential of sub-nano materials down to the single atom level. The behavior of materials has been shown to change as the materials get significantly smaller. This new knowledge could lead to the further development of advanced materials and the greater miniaturization of devices—miniaturization being the calling card of electronic medical devices today.
“This system that PSI will be launching for sale in the next few months is capable of measuring the properties of a single atom of material,” Murak told Medical Product Outsourcing. “The technology that’s out there right now can accommodate 5-10 nanometer sizes. We all hear of nano being ‘hot.’ This is sub-nano. For things to continue to advance, the world needs the measurement tools to understand the properties of a material when they get significantly smaller than the 5-10 nanometer range. What this is opening the door to is for the developer of a pacemaker to make a pacemaker the size of a pinhead, for instance. What happens when materials get ultra small is that the properties change. The properties of gold, for example, have been found and proven to change when they get down to the single atom in size. This really opens up the door for the development of new devices and new materials.”
Indeed, gold does behave differently as a single atom in comparison when it exists in multi-atom molecules. And gold being an inert metal, it is very commonly used in electronic medical devices, especially implantable ones. However, as a single atom, its behavior can be very volatile, according to nanotechnology researchers Earl Boysen and Nancy Muir Boysen. As a single atom, it can act as a catalyst to the oxidization of carbon monoxide, for instance. The smaller the nanoparticle, the larger the proportion of atoms at the surface, and the larger proportion of atoms at the corners of the crystal. While in the bulk form, each gold atom (except the small percentage of them at the surface) is surrounded by 12 other gold atoms. Even the gold atoms at the surface of the molecule each have six adjacent gold atoms. In a smaller gold nanoparticle, a much larger percentage of gold atoms sit at the surface. Because gold forms crystalline shapes, gold atoms at the corners of the crystals are surrounded by fewer gold atoms than those in the surface of bulk gold. The atoms at the corners of the crystal have much more exposed surface area and therefore are more reactive than gold atoms in the bulk form, which allows the gold nanoparticles to catalyze reactions.
PSI is finding innovative ways to use the inherent properties of materials in their atomic states. Espousing Saint Exupéry’s version of perfection really is as simple as stepping back and letting materials speak (and act) for themselves. But of course, the tools that surround these advancements—measurement, analysis and manipulation equipment—are anything but simple.
Making the Connection
Addressing that data-transfer problem has not been easy for smartphone manufacturers and cell service providers. Wireless communication is still far from perfectly reliable, and it also is vulnerable to all manner of interference, both unintentional and malicious. Wired data transfer, too, is subject to all manner of interference and attacks. When it comes to electronic medical devices, security becomes a matter of life and death.
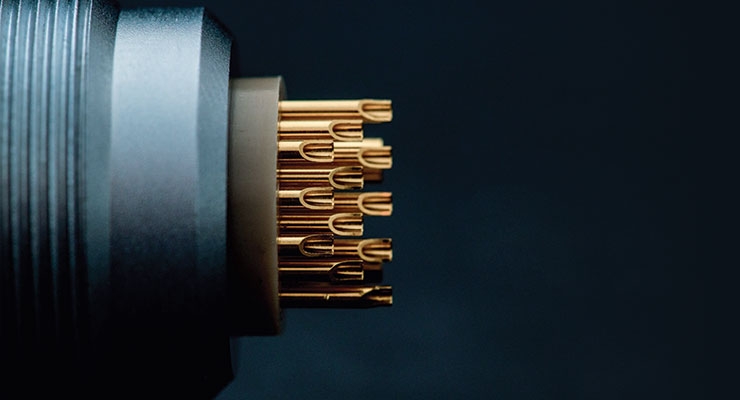
Companies such as ODU-USA Inc. are focused solely on connectors, both electrical and data transfer, and therefore can perfect them according to the application called for. Paul Kawamoto, push-pull product manager for the Camarillo, Calif.-based company, noted to MPO that there is a lot of industry discussion surrounding USB 3.1 requirements versus the type of connector tech giant Apple Inc. uses, the Thunderbolt. USB 3.1 is the ninth iteration of the universal serial bus (USB) standard for interfacing computers and electronic devices. USB 3.0 added the new transfer mode SuperSpeed that could transfer data at up to 5 gigabits per second, more than ten times faster than the USB 2.0 standard. USB 3.1 transfers data at up to 10 gigabits per second. Thunderbolt, developed under the name Light Peak, also is a hardware interface that allows the connection of external peripherals to a computer. Thunderbolt soon will be released in its third iteration, transferring data up to 40 gigabits per second. USB, however, is still by far the cheaper option.
These connectors are the tiny point of connection between data and the data’s destination, and therefore are vital components in a world of medical devices that is becoming increasingly mobile, portable and wearable. And if patients are now carrying their health devices, including biometrics measurement devices such as continuous glucose monitors, bad data transfer could have detrimental consequences.
But don’t be fooled. The connector options don’t end at USB vs. Thunderbolt. Lemo USA Inc., a Rohnert Park, Calif.-based provider of precision custom connection and cable solutions, has more than 75,000 combinations of connectors available.
“Many of our medical customers can find a catalog connector that will fit their application very nicely,” said Lemo Products and Apps Engineer Randy Jew. “When a custom connector is needed, it’s usually a modification of one of the attributes such as the shape, latching, collet termination, or insert configuration. Lemo has come out with both smaller size connectors to accommodate smaller devices, and higher density inserts to accommodate smaller contacts which gives higher pin counts. We have also seen a need for a disposable type lower-cost connector which we were able to design the Single Patient Use connector series for these medical OEMs (original equipment manufacturers).”
Companies such as ODU have to follow in the wake of medical device OEMs demanding smaller and smaller devices with the same or greater capabilities. As makers of connectors, they are only one cog in the machine of custom electronic components—but a vital one. What would an iPhone be without its Thunderbolt, through which we transfer and store our music and photos? Fischer Connectors Inc., which has U.S. offices are in Alpharetta, Ga., provides various custom options specialized for medical applications.
“Everything is getting smaller and smarter, and it is no different for connector solutions,” Fischer’s President and CEO Stephen J. Gosk told MPO. “We have a new rugged miniature connector that handles both signal and power to help our customers build smaller devices. For medical applications, we now have very small stainless steel connectors and silicone overmolds that are appropriate for high heat sterilization. The smarter part means moving more data faster, which is a combination of the connector and the cable. We have done a lot of research for our customers to help provide them with the right combination that gets the data to where it needs to go quickly and accurately. We are even seeing people integrate fiber optics into their medical devices and facilities so that the data can move farther and faster.”
“Achieving data rate and data speed, while maintaining excellent quality and that type of performance to the end user, while obviously minimizing the size and weight of the device — those are some big challenges,” Kawamoto added. “But over the past five or six years, the advances have improved tremendously. I think technology will just continue to advance, and we’ll be shocked at what we see next ten years from now.”
Finding The Line
Let us not forget, however, that finding and surfing that perfect wave crest of miniaturized devices with added technology features to maintain capabilities is still a worthwhile endeavor. Indeed, it is how the medtech industry provides its most forward-thinking, life-saving technologies.
Sachseln, Switzerland-based maxon Precision Motors Inc., which makes high-precision drive systems, constantly must find ways to provide the same level of motor power while still fitting within the ever-smaller medical devices and equipment from its OEM customers. Motion Control Engineering Manager Biren Patel recalled recent challenge the company met.
“Recently, in a prosthetic application, we had to shrink down our position controller to fit within the prosthesis and deliver up to 40 amperes peak of output current,” Patel explained to MPO. “One of the things we’ve done internally to overcome that is we’ve developed an ASIC [application-specific integrated circuit] which combines more than 50 discrete components and miniaturizes that into one chip, so there’s a lot of real estate saved there. Furthermore, a sophisticated thermal design with improved MOSFETs [metal–oxide–semiconductor field-effect transistors, which drive power] and printed circuit board technologies was the key on that front to miniaturize the overall footprint of the board.”
“In general, devices are smaller and more powerful,” SoPark’s Murak said. “The biometric devices we produce circuit board assemblies for now can be the size of a silver dollar, and it’s amazing how much data they can gather off someone related to their health and wellbeing.”
President and Chief Operating Officer of SoPark Rupa Shanmugam, who has an extensive background in electrical engineering, noted that in order to keep up with this miniaturization trend, the company “has to keep investing in new equipment that can support that type of component placement accuracy as well.” Some components SoPark works with are microchips the size of a grain of salt. A recently acquired piece of equipment on the company’s manufacturing floor can place up to 60,000 of these components in one hour. Previously, they were only able to place about 15,000 at a time.
“But way ahead of every OEM demand is quality,” Murak concluded. “The ability to have a solid quality assurance system is something OEMs are tremendously interested in. Does a company like SoPark have good quality management system in place? They want to know if you have all your ducks in a row, things are in order. Not only should they be well documented, but there should be good process control and good root cause analysis. These demands drove us to traceability at the component level.
Certainly an implication of all this miniaturization in the medical device industry is traceability. It is imperative you know where a component came from, who placed it on the circuit board, how and when, and so on. When the component is size of a grain of salt, that’s no small undertaking.”
No, really. If applying for a work-from-home position, most companies will insist you have a land line. Without one, telecommunications are unreliable; and even if your cell phone service carrier is the most reliable in the world, the basic sound quality still leaves a lot to be desired.
In a 2014 article cheekily titled “All Smart, No Phone,” Spectrum explained that cell phone carriers face several obstacles to achieving better voice quality standards. The first is simply an aging public switched telephone network. The public switched telephone network (PSTN) is the world’s collection of interconnected voice-oriented public telephone networks, both commercial and government-owned. It’s also referred to as the plain old telephone service (POTS). The second is hardware design. The original Motorola “brick” phones, one time a status symbol and now, a mere 30 years later, a joke, were constructed with voice calls in mind. They had large speakers about the size of an ear, and the microphone pointed directly towards the mouth. Which is easier to do with a mobile phone the size of an actual brick as opposed to one the size of a business card.
Chris Kyriakakis, founder and chief technology officer of Audyssey, a Los Angeles, Calif.–based acoustic design company, said the modern smartphone’s form “is driven by industrial design and not voice quality.” The screens are flat to facilitate scrolling, paging, thumbing, and all kinds of visual-targeted applications. The phone’s body is shaped and sized to fit as easily into the pocket as it does in the hand. This leaves little room for speaker components, which are compressed, dampening frequencies, making the richest of voices sound tinny and thin. Smartphone designers then add software to lessen such distortions. The microphone poses even more problems. For one, it doesn’t even face the mouth anymore. And one doesn’t need a degree in audiology to know that the further away a mic is from the mouth, the more background noise it picks up. Higher-end smartphones try to address this problem by using multiple microphones. The one closest to the mouth focus on the voice, while the ones further away compare sounds to the first mic to identify and then filter out unwanted sounds.
But in his seminal (and surrealist) text “The Little Prince,” Antoine de Saint Exupéry said, “Perfection is achieved not when there is nothing more to add, but when there is nothing left to take away.” Shaving down the smartphone to a more minimal, small, portable, wearable, pocketable, mobile form, and then adding features to it to make up for what was lost, can start to seem a little surreal in itself. This is not to pooh-pooh technological advancement. It is indeed quite a feat of modern science that we can carry around a device that allows us to communicate in voice and text form, watch video, listen to music, play games, manage our schedules and monitor our health in our pockets. But there must be a critical point at which miniaturization and technological add-ons converge and cease to advance.
Right?
The Golden Balance
Getting medical device components down to the “nano” (nanometer, or nm) level is pretty hip right now. At least, the term gets thrown around a lot. Just in July, Mequon, Wis.-based spine implant surface technology company Titan Spine hired a specialist to address precisely this problem: namely, that the notion of nanotechnology is not fully understood by the medical device industry. “One of Jim [Sevey’s] initial tasks will be to clearly differentiate the science of our nanotechnology platform from those that claim to have nanotechnology but have not been cleared by the FDA to do so,” Titan Spine President Kevin Gemas said of the company’s new hire.
But Gerry Murak is past nano. The CEO of Buffalo, N.Y.-based electronic contract manufacturer of printed circuit board assemblies, cables, wiring harness and electro-mechanical assemblies SoPark Corporation, which is ISO 13485 certified, has entered into a new venture with the State University of New York at Buffalo. Three researchers at the school have invented a disruptive technology that studies materials at the atomic level. Murak founded Precision Scientific Instruments Inc. (PSI) to license the technology that is touted to be able to examine materials at 50 times greater resolution than any other technology on the market.
“Sub-nano devices are the new frontier of state-of-the-art nano technology,” Murak claimed.
The PSi1 system, as it’s called, is hoped to allow researchers to unlock the commercial potential of sub-nano materials down to the single atom level. The behavior of materials has been shown to change as the materials get significantly smaller. This new knowledge could lead to the further development of advanced materials and the greater miniaturization of devices—miniaturization being the calling card of electronic medical devices today.
“This system that PSI will be launching for sale in the next few months is capable of measuring the properties of a single atom of material,” Murak told Medical Product Outsourcing. “The technology that’s out there right now can accommodate 5-10 nanometer sizes. We all hear of nano being ‘hot.’ This is sub-nano. For things to continue to advance, the world needs the measurement tools to understand the properties of a material when they get significantly smaller than the 5-10 nanometer range. What this is opening the door to is for the developer of a pacemaker to make a pacemaker the size of a pinhead, for instance. What happens when materials get ultra small is that the properties change. The properties of gold, for example, have been found and proven to change when they get down to the single atom in size. This really opens up the door for the development of new devices and new materials.”
Indeed, gold does behave differently as a single atom in comparison when it exists in multi-atom molecules. And gold being an inert metal, it is very commonly used in electronic medical devices, especially implantable ones. However, as a single atom, its behavior can be very volatile, according to nanotechnology researchers Earl Boysen and Nancy Muir Boysen. As a single atom, it can act as a catalyst to the oxidization of carbon monoxide, for instance. The smaller the nanoparticle, the larger the proportion of atoms at the surface, and the larger proportion of atoms at the corners of the crystal. While in the bulk form, each gold atom (except the small percentage of them at the surface) is surrounded by 12 other gold atoms. Even the gold atoms at the surface of the molecule each have six adjacent gold atoms. In a smaller gold nanoparticle, a much larger percentage of gold atoms sit at the surface. Because gold forms crystalline shapes, gold atoms at the corners of the crystals are surrounded by fewer gold atoms than those in the surface of bulk gold. The atoms at the corners of the crystal have much more exposed surface area and therefore are more reactive than gold atoms in the bulk form, which allows the gold nanoparticles to catalyze reactions.
PSI is finding innovative ways to use the inherent properties of materials in their atomic states. Espousing Saint Exupéry’s version of perfection really is as simple as stepping back and letting materials speak (and act) for themselves. But of course, the tools that surround these advancements—measurement, analysis and manipulation equipment—are anything but simple.
Making the Connection
Addressing that data-transfer problem has not been easy for smartphone manufacturers and cell service providers. Wireless communication is still far from perfectly reliable, and it also is vulnerable to all manner of interference, both unintentional and malicious. Wired data transfer, too, is subject to all manner of interference and attacks. When it comes to electronic medical devices, security becomes a matter of life and death.
Companies such as ODU-USA Inc. are focused solely on connectors, both electrical and data transfer, and therefore can perfect them according to the application called for. Paul Kawamoto, push-pull product manager for the Camarillo, Calif.-based company, noted to MPO that there is a lot of industry discussion surrounding USB 3.1 requirements versus the type of connector tech giant Apple Inc. uses, the Thunderbolt. USB 3.1 is the ninth iteration of the universal serial bus (USB) standard for interfacing computers and electronic devices. USB 3.0 added the new transfer mode SuperSpeed that could transfer data at up to 5 gigabits per second, more than ten times faster than the USB 2.0 standard. USB 3.1 transfers data at up to 10 gigabits per second. Thunderbolt, developed under the name Light Peak, also is a hardware interface that allows the connection of external peripherals to a computer. Thunderbolt soon will be released in its third iteration, transferring data up to 40 gigabits per second. USB, however, is still by far the cheaper option.
These connectors are the tiny point of connection between data and the data’s destination, and therefore are vital components in a world of medical devices that is becoming increasingly mobile, portable and wearable. And if patients are now carrying their health devices, including biometrics measurement devices such as continuous glucose monitors, bad data transfer could have detrimental consequences.
But don’t be fooled. The connector options don’t end at USB vs. Thunderbolt. Lemo USA Inc., a Rohnert Park, Calif.-based provider of precision custom connection and cable solutions, has more than 75,000 combinations of connectors available.
“Many of our medical customers can find a catalog connector that will fit their application very nicely,” said Lemo Products and Apps Engineer Randy Jew. “When a custom connector is needed, it’s usually a modification of one of the attributes such as the shape, latching, collet termination, or insert configuration. Lemo has come out with both smaller size connectors to accommodate smaller devices, and higher density inserts to accommodate smaller contacts which gives higher pin counts. We have also seen a need for a disposable type lower-cost connector which we were able to design the Single Patient Use connector series for these medical OEMs (original equipment manufacturers).”
Companies such as ODU have to follow in the wake of medical device OEMs demanding smaller and smaller devices with the same or greater capabilities. As makers of connectors, they are only one cog in the machine of custom electronic components—but a vital one. What would an iPhone be without its Thunderbolt, through which we transfer and store our music and photos? Fischer Connectors Inc., which has U.S. offices are in Alpharetta, Ga., provides various custom options specialized for medical applications.
“Everything is getting smaller and smarter, and it is no different for connector solutions,” Fischer’s President and CEO Stephen J. Gosk told MPO. “We have a new rugged miniature connector that handles both signal and power to help our customers build smaller devices. For medical applications, we now have very small stainless steel connectors and silicone overmolds that are appropriate for high heat sterilization. The smarter part means moving more data faster, which is a combination of the connector and the cable. We have done a lot of research for our customers to help provide them with the right combination that gets the data to where it needs to go quickly and accurately. We are even seeing people integrate fiber optics into their medical devices and facilities so that the data can move farther and faster.”
“Achieving data rate and data speed, while maintaining excellent quality and that type of performance to the end user, while obviously minimizing the size and weight of the device — those are some big challenges,” Kawamoto added. “But over the past five or six years, the advances have improved tremendously. I think technology will just continue to advance, and we’ll be shocked at what we see next ten years from now.”
Finding The Line
Let us not forget, however, that finding and surfing that perfect wave crest of miniaturized devices with added technology features to maintain capabilities is still a worthwhile endeavor. Indeed, it is how the medtech industry provides its most forward-thinking, life-saving technologies.
Sachseln, Switzerland-based maxon Precision Motors Inc., which makes high-precision drive systems, constantly must find ways to provide the same level of motor power while still fitting within the ever-smaller medical devices and equipment from its OEM customers. Motion Control Engineering Manager Biren Patel recalled recent challenge the company met.
“Recently, in a prosthetic application, we had to shrink down our position controller to fit within the prosthesis and deliver up to 40 amperes peak of output current,” Patel explained to MPO. “One of the things we’ve done internally to overcome that is we’ve developed an ASIC [application-specific integrated circuit] which combines more than 50 discrete components and miniaturizes that into one chip, so there’s a lot of real estate saved there. Furthermore, a sophisticated thermal design with improved MOSFETs [metal–oxide–semiconductor field-effect transistors, which drive power] and printed circuit board technologies was the key on that front to miniaturize the overall footprint of the board.”
“In general, devices are smaller and more powerful,” SoPark’s Murak said. “The biometric devices we produce circuit board assemblies for now can be the size of a silver dollar, and it’s amazing how much data they can gather off someone related to their health and wellbeing.”
President and Chief Operating Officer of SoPark Rupa Shanmugam, who has an extensive background in electrical engineering, noted that in order to keep up with this miniaturization trend, the company “has to keep investing in new equipment that can support that type of component placement accuracy as well.” Some components SoPark works with are microchips the size of a grain of salt. A recently acquired piece of equipment on the company’s manufacturing floor can place up to 60,000 of these components in one hour. Previously, they were only able to place about 15,000 at a time.
“But way ahead of every OEM demand is quality,” Murak concluded. “The ability to have a solid quality assurance system is something OEMs are tremendously interested in. Does a company like SoPark have good quality management system in place? They want to know if you have all your ducks in a row, things are in order. Not only should they be well documented, but there should be good process control and good root cause analysis. These demands drove us to traceability at the component level.
Certainly an implication of all this miniaturization in the medical device industry is traceability. It is imperative you know where a component came from, who placed it on the circuit board, how and when, and so on. When the component is size of a grain of salt, that’s no small undertaking.”