07.21.20
Rank: #27 (Last year: #27) $3.36 Billion
Prior Fiscal: $3.21 Billion
Percentage Change: +4.6%
No. of Employees: 6,478
Global Headquarters: Marlborough, Mass.
KEY EMPLOYEES:
Stephen P. MacMillan, Chairman, President, and CEO
Jay A. Stein, Co-founder, Chairman Emeritus, Sr. VP, Chief Technical Officer
Monica Aguirre Berthelot, Chief of Staff
Patrick Bray, Sr. VP, Global Supply Chain, Quality and Regulatory
Sean S. Daugherty, Division President, GYN Surgical Solutions
John M. Griffin, General Counsel
Karleen Oberton, Chief Financial Officer
Sanjay Prabhakaran, Regional President, Asia Pacific
Kevin R. Thornal, Division President, Diagnostic Solutions
Peter J. Valenti III, Division President, Breast and Skeletal Health Solutions
Jan Verstreken, Regional President, EMEA and Canada
The odds were not good.
Actually, they were worse than that: They were awful. Downright frightful, even.
Lindsay was aware of the odds. She was cognizant of the tainted family history (both sides) that made her more vulnerable than others to breast and ovarian cancers. She knew about the maternal aunts that succumbed to malignancies, one of them being just 22.
Lindsay watched her mother battle Stage 0 breast cancer. She was well-versed in BRCA1 and BRCA2 knowledge, and had heard about Hereditary Breast and Ovarian Cancer Syndrome (HBOC).
Indeed, Lindsay was keenly aware of the risks she faced from her genetic family tree. Hence her request (more like a fight, really) for a mammogram at age 35—10 years before the recommended cancer screening age for average-risk women and five years before the suggested age for higher-risk females.
Lindsay’s first mammogram was normal. But as her second screening approached, she discovered a lump, prompting her to dutifully schedule a diagnostic mammogram and ultrasound. Only one test was needed, however, to confirm Lindsay’s worst fears.
“Before I got to do the ultrasound, they asked me to come back in for more mammogram pictures,” the mother of two recalls in a YouTube video. “I was like, ‘oh s*it, this is not good.’ The doctor came in and told me right then that it was a 99 percent chance that this was cancer. I just was in shock. Like, I couldn’t die.”
It wasn’t death that Lindsay was afraid of, though. Rather, she was frightened to leave her husband and two young children, ages 5 and 7 at the time of diagnosis.
Fortunately, doctors caught Lindsay’s cancer early and she successfully beat the disease, becoming one of the few survivors to overcome her doomed destiny. Though Lindsay’s mother Deidre credits her daughter’s survival to determination and persistence, Lindsay attributes her success to the 3D mammogram she underwent after finding the lump.
ANALYST INSIGHTS: Hologic had some timely COVID related portfolio platforms that allowed it to be “opportunistic” in creating growth during a quarter (Q2 2020) in which it might ordinarily have had a difficult time. With more strength in its diagnostic business now established, expect Hologic to combine that momentum with its Women’s Health position to find momentum for a positive 2020/2021.
3D mammograms combine multiple breast X-rays to create a three-dimensional picture of the breast. While they generally are used to detect cancer in patients with no signs or symptoms, 3D mammograms also can help clinicians investigate the cause of problems like breast masses, pain, and nipple discharge.
3D mammogram machines create both 3D and standard two-dimensional breast images. On average, 3D screenings may slightly increase cancer detection rates, finding about one extra breast tumor for every 1,000 U.S. women screened, according to a 2018 analysis in the Journal of the National Cancer Institute. Most studies also show that 3D screenings cause fewer “false alarms,” in which women are called back for procedures they don’t need. But more research is needed to determine whether 3D mammograms reduce breast cancer death risk significantly more than their two-dimensional counterparts alone.
Either way, Lindsay is grateful to have had access to 3D mammography. “I feel extremely lucky that I had the 3D mammogram accessible to me,” she said in the YouTube video. “I was able to be diagnosed quickly and I was still early stage. And that really saved my life.”
3D mammography also has been a lifesaver of sorts for Marlborough, Mass.-based Hologic Inc., which has installed nearly 7,000 three-dimensional imaging systems throughout the United States. Stronger sales of the company’s 3Dimensions and 3D Performance systems in FY19 improved total revenue 4.6 percent to $3.36 billion and helped drive a 10.3 percent surge in Breast Health product proceeds.
“Our core 3D mammography business remains rock solid and we are building on it with an increasingly diversified product portfolio that spans the continuum of Breast Health care. In U.S. Breast Health, we are using internal R&D and external acquisitions to build on an incredibly strong domestic leadership position in 3D mammography,” Hologic Chairman, president, and CEO Stephen P. MacMillan told investors on a fourth-quarter earnings call last fall. “By leveraging our installed base we are creating a steadier more diversified growth engine across the continuum of breast healthcare.”
Fueling that engine in FY19 was the previous fiscal year’s purchases of Faxitron Bioptics and Focal Therapeutics, which together contributed $44.7 million to the Breast Health division’s $758.5 million total. Other growth stokers included newer workflow products like Intelligent 2D, Clarity HD, and SmartCurve upgrades, though gains were somewhat stymied by fluctuating foreign currency rates, 3D software upgrades, and lower sales of Brevera breast biopsy systems (supply constraints), Affirm Prone Breast Biopsy tables, and various software features.
Similar forces were at play in Hologic’s Skeletal Health division, where the Faxitron and Focal integration combined with higher demand for Horizon DXA systems to bump up sales 5.1 percent to $65.5 million. In FY19, the division garnered 74.6 percent of its product revenue in the United States, 12 percent in Europe, 8.8 percent in Asia-Pacific, and 4.6 percent in other international markets.
Diagnostics more or less mimicked that geographic sales apportionment in FY19, generating 76.4 percent of sales in the United States and 23.6 percent internationally. Total division revenue rose 5 percent to $1.17 billion, driven largely by a $64.2 million uptick in Molecular Diagnostics sales (excluding blood screening products). That uptick was triggered by a $41.9 million increase in worldwide Aptima assay sales (itself fueled by an expanded Panther instrument base), as well as robust demand for virology and Fusion devices.
Hologic expanded its Aptima and Fusion portfolios during the fiscal year with regulatory approvals in the both the United States and Europe. In October 2018, the company won CE marking for its Panther Fusion Bordetella Assay, a real-time PCR test for detecting and differentiating Bordetella pertussis and Bordetella parapertussis, the bacteria most commonly associated with Whooping cough.
Four months after that approval (February 2019), Hologic received two CE marks for its Aptima HIV-1 Quant Dx Assay for use in testing dried blood spots and early diagnosis of the disease in infants. The two CE marks gave the company the right to use the assay in European and African countries to qualitatively detect HIV type 1 RNA in infants younger than 18 months. Clinicians in those countries also can use the assay in testing dried blood spots to monitor viral load and disease progression in HIV-1 infected patients.
Stateside consent came last spring with the U.S. Food and Drug Administration (FDA) clearances of the Aptima BV assay for bacterial vaginosis detection and the Aptima CV/TV assay for Candida vaginitis and Trichomonas vaginalis identification. “Vaginitis is one of the most common reasons women visit a healthcare provider, and Hologic’s new molecular assays have the potential to transform how these infections are diagnosed in that very first appointment,” Edward Evantash, M.D., medical director and vice president of medical affairs, Hologic, said in publicizing the clearances. “The improved sensitivity and specificity of Hologic’s molecular assays over traditional methods in determining the underlying cause of vaginitis not only means identifying the right infection, but enabling the right treatment and, in turn, reducing the potential for recurrent or persistent infections.”
Aptima’s two U.S. authorizations raised Hologic’s FDA-cleared assay total to 16.
Hologic expanded its Cytology & Perinatal product platform as well in fiscal 2019 (ended Sept. 28, 2019), but the new additions failed to plug an $8.6 million deficit stemming from lower Perinatal volumes. A number of factors reduced Perinatal demand, including a shift in ordering patterns and lower domestic ThinPrep test volume; intriguingly though, the company’s ThinPrep test portfolio received a boost overseas with the CE marking last April of the ThinPrep Genesis processor, an instrument that prepares slides for cytology and aliquot samples for molecular testing. Compared to older instruments, the ThinPrep Genesis processor has increased automation capabilities and provides ergonomic and chain-of-custody benefits. It also provides automated sample barcoding, helping to ensure accurate sample tracking and reducing manual steps.
Hologic attributed the lower ThinPrep test volumes in the United States to screening interval expansion and a decline in average selling prices.
The Cytology & Perinatal product lineup was not the only unprofitable portfolio, though. Women’s Health finished fiscal 2019 with a deficit due to lower MonaLisa Touch gynecologic laser sales, which fell victim to an FDA crackdown on “vaginal rejuvenation” devices. These products use lasers and other energy-based devices to remove or reshape vaginal tissue in an effort to treat conditions and symptoms of menopause, urinary incontinence, or sexual function. But clinical evidence on such devices is lacking, and the FDA has identified numerous cases of vaginal burns or scarring tied to vaginal rejuvenation, as well as post-procedural pain during sexual intercourse or recurring chronic pain.
As a result of the FDA’s concerns, Hologic temporarily suspended sales of its TempSure Vitalia handpieces and single-use probes. The products re-entered the market in Q1 of fiscal 2019.
The loss in Women’s Health sales was partly responsible for the FY19 deficit in Medical Aesthetics product revenue. Other contributors to the 9.2 percent sales decrease (to $252.9 million) included lower body contouring product demand; fewer sales of SculpSure lasers, Submental upgrades, and related PAC keys, increased competition in non-invasive fat-reduction technology, and fluctuating foreign currency exchange rates.
Offsetting the Medical Aesthetics division’s profit-busters was higher skin product revenue, driven mainly by robust Icon and TempSure system sales.
Similarly, increased MyoSure and Fluent systems volume helped boost GYN Surgical product revenue 3.6 percent in FY19 to $436.2 million. MyoSure contributed $9.7 million to the total, while Fluent chipped in $10.8 million. Also supporting GYN Surgical’s overall sales increase was the first-quarter U.S. launch of the Omni hysteroscope, a three-in-one modular scope with advanced visualization capabilities designed for both diagnostic and therapeutic procedures. Approved by Canadian and European regulators in March 2019, the Omni scope can be used in doctors’ offices, surgical centers, or operating rooms.
The increases realized through Fluent, MyoSure, and Omni sales were partially neutralized by a $14 million loss in NovaSure systems revenue. Hologic attributed that shortfall to increased competition, a stagnant market for endometrial ablation, and lower average selling prices.
Another profit-maker for Hologic in fiscal 2019 was its Service and Other Revenues division, comprised primarily of sales generated by the firm’s field service organization for ongoing product service, installation, and repair. Most of the proceeds in this division come from the Breast Health segment, and to a lesser extent, the Medical Aesthetics business. Service and Other Revenues rose 3.8 percent to $596 million on higher installation rates, spare parts, and service contract conversion and license renewal rates for the Breast Health business.
Hologic bolstered the Breast Health business in FY19 with its purchase of a 46 percent stake in French firm SuperSonic Imagine. SuperSonic’s products complement Hologic’s wireless, handheld ultrasound scanner Viera, which is commercialized through a development and distribution agreement with Clarius Mobile Health.
Upon purchase of the SuperSonic stake, MacMillan said: “SuperSonic Imagine provides best-in-class ultrasound technology that is strategically important to the long-term growth of our Breast Health business.”
A slew of product launches and approvals throughout FY19 helped foster the growth of Hologic’s other businesses. Those actions included:
COVID-19 Consequences
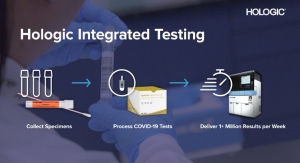
Two months after securing that authorization, Hologic won a second EUA from the FDA (May 15) for its Aptima SARS-CoV-2 virus detection assay. This test runs on Hologic’s fully automated Panther system, more than 1,000 of which are installed in U.S. clinical laboratories. Each Panther system provides initial results in roughly three hours and can process more than 1,000 COVID-19 tests in 24 hours. More than 1,800 Panther systems have been installed in 60 countries, according to the company.
Prior Fiscal: $3.21 Billion
Percentage Change: +4.6%
No. of Employees: 6,478
Global Headquarters: Marlborough, Mass.
KEY EMPLOYEES:
Stephen P. MacMillan, Chairman, President, and CEO
Jay A. Stein, Co-founder, Chairman Emeritus, Sr. VP, Chief Technical Officer
Monica Aguirre Berthelot, Chief of Staff
Patrick Bray, Sr. VP, Global Supply Chain, Quality and Regulatory
Sean S. Daugherty, Division President, GYN Surgical Solutions
John M. Griffin, General Counsel
Karleen Oberton, Chief Financial Officer
Sanjay Prabhakaran, Regional President, Asia Pacific
Kevin R. Thornal, Division President, Diagnostic Solutions
Peter J. Valenti III, Division President, Breast and Skeletal Health Solutions
Jan Verstreken, Regional President, EMEA and Canada
The odds were not good.
Actually, they were worse than that: They were awful. Downright frightful, even.
Lindsay was aware of the odds. She was cognizant of the tainted family history (both sides) that made her more vulnerable than others to breast and ovarian cancers. She knew about the maternal aunts that succumbed to malignancies, one of them being just 22.
Lindsay watched her mother battle Stage 0 breast cancer. She was well-versed in BRCA1 and BRCA2 knowledge, and had heard about Hereditary Breast and Ovarian Cancer Syndrome (HBOC).
Indeed, Lindsay was keenly aware of the risks she faced from her genetic family tree. Hence her request (more like a fight, really) for a mammogram at age 35—10 years before the recommended cancer screening age for average-risk women and five years before the suggested age for higher-risk females.
Lindsay’s first mammogram was normal. But as her second screening approached, she discovered a lump, prompting her to dutifully schedule a diagnostic mammogram and ultrasound. Only one test was needed, however, to confirm Lindsay’s worst fears.
“Before I got to do the ultrasound, they asked me to come back in for more mammogram pictures,” the mother of two recalls in a YouTube video. “I was like, ‘oh s*it, this is not good.’ The doctor came in and told me right then that it was a 99 percent chance that this was cancer. I just was in shock. Like, I couldn’t die.”
It wasn’t death that Lindsay was afraid of, though. Rather, she was frightened to leave her husband and two young children, ages 5 and 7 at the time of diagnosis.
Fortunately, doctors caught Lindsay’s cancer early and she successfully beat the disease, becoming one of the few survivors to overcome her doomed destiny. Though Lindsay’s mother Deidre credits her daughter’s survival to determination and persistence, Lindsay attributes her success to the 3D mammogram she underwent after finding the lump.
ANALYST INSIGHTS: Hologic had some timely COVID related portfolio platforms that allowed it to be “opportunistic” in creating growth during a quarter (Q2 2020) in which it might ordinarily have had a difficult time. With more strength in its diagnostic business now established, expect Hologic to combine that momentum with its Women’s Health position to find momentum for a positive 2020/2021.
—Dave Sheppard, Co-Founder and Managing Director, MedWorld Advisors
3D mammograms combine multiple breast X-rays to create a three-dimensional picture of the breast. While they generally are used to detect cancer in patients with no signs or symptoms, 3D mammograms also can help clinicians investigate the cause of problems like breast masses, pain, and nipple discharge.
3D mammogram machines create both 3D and standard two-dimensional breast images. On average, 3D screenings may slightly increase cancer detection rates, finding about one extra breast tumor for every 1,000 U.S. women screened, according to a 2018 analysis in the Journal of the National Cancer Institute. Most studies also show that 3D screenings cause fewer “false alarms,” in which women are called back for procedures they don’t need. But more research is needed to determine whether 3D mammograms reduce breast cancer death risk significantly more than their two-dimensional counterparts alone.
Either way, Lindsay is grateful to have had access to 3D mammography. “I feel extremely lucky that I had the 3D mammogram accessible to me,” she said in the YouTube video. “I was able to be diagnosed quickly and I was still early stage. And that really saved my life.”
3D mammography also has been a lifesaver of sorts for Marlborough, Mass.-based Hologic Inc., which has installed nearly 7,000 three-dimensional imaging systems throughout the United States. Stronger sales of the company’s 3Dimensions and 3D Performance systems in FY19 improved total revenue 4.6 percent to $3.36 billion and helped drive a 10.3 percent surge in Breast Health product proceeds.
“Our core 3D mammography business remains rock solid and we are building on it with an increasingly diversified product portfolio that spans the continuum of Breast Health care. In U.S. Breast Health, we are using internal R&D and external acquisitions to build on an incredibly strong domestic leadership position in 3D mammography,” Hologic Chairman, president, and CEO Stephen P. MacMillan told investors on a fourth-quarter earnings call last fall. “By leveraging our installed base we are creating a steadier more diversified growth engine across the continuum of breast healthcare.”
Fueling that engine in FY19 was the previous fiscal year’s purchases of Faxitron Bioptics and Focal Therapeutics, which together contributed $44.7 million to the Breast Health division’s $758.5 million total. Other growth stokers included newer workflow products like Intelligent 2D, Clarity HD, and SmartCurve upgrades, though gains were somewhat stymied by fluctuating foreign currency rates, 3D software upgrades, and lower sales of Brevera breast biopsy systems (supply constraints), Affirm Prone Breast Biopsy tables, and various software features.
Similar forces were at play in Hologic’s Skeletal Health division, where the Faxitron and Focal integration combined with higher demand for Horizon DXA systems to bump up sales 5.1 percent to $65.5 million. In FY19, the division garnered 74.6 percent of its product revenue in the United States, 12 percent in Europe, 8.8 percent in Asia-Pacific, and 4.6 percent in other international markets.
Diagnostics more or less mimicked that geographic sales apportionment in FY19, generating 76.4 percent of sales in the United States and 23.6 percent internationally. Total division revenue rose 5 percent to $1.17 billion, driven largely by a $64.2 million uptick in Molecular Diagnostics sales (excluding blood screening products). That uptick was triggered by a $41.9 million increase in worldwide Aptima assay sales (itself fueled by an expanded Panther instrument base), as well as robust demand for virology and Fusion devices.
Hologic expanded its Aptima and Fusion portfolios during the fiscal year with regulatory approvals in the both the United States and Europe. In October 2018, the company won CE marking for its Panther Fusion Bordetella Assay, a real-time PCR test for detecting and differentiating Bordetella pertussis and Bordetella parapertussis, the bacteria most commonly associated with Whooping cough.
Four months after that approval (February 2019), Hologic received two CE marks for its Aptima HIV-1 Quant Dx Assay for use in testing dried blood spots and early diagnosis of the disease in infants. The two CE marks gave the company the right to use the assay in European and African countries to qualitatively detect HIV type 1 RNA in infants younger than 18 months. Clinicians in those countries also can use the assay in testing dried blood spots to monitor viral load and disease progression in HIV-1 infected patients.
Stateside consent came last spring with the U.S. Food and Drug Administration (FDA) clearances of the Aptima BV assay for bacterial vaginosis detection and the Aptima CV/TV assay for Candida vaginitis and Trichomonas vaginalis identification. “Vaginitis is one of the most common reasons women visit a healthcare provider, and Hologic’s new molecular assays have the potential to transform how these infections are diagnosed in that very first appointment,” Edward Evantash, M.D., medical director and vice president of medical affairs, Hologic, said in publicizing the clearances. “The improved sensitivity and specificity of Hologic’s molecular assays over traditional methods in determining the underlying cause of vaginitis not only means identifying the right infection, but enabling the right treatment and, in turn, reducing the potential for recurrent or persistent infections.”
Aptima’s two U.S. authorizations raised Hologic’s FDA-cleared assay total to 16.
Hologic expanded its Cytology & Perinatal product platform as well in fiscal 2019 (ended Sept. 28, 2019), but the new additions failed to plug an $8.6 million deficit stemming from lower Perinatal volumes. A number of factors reduced Perinatal demand, including a shift in ordering patterns and lower domestic ThinPrep test volume; intriguingly though, the company’s ThinPrep test portfolio received a boost overseas with the CE marking last April of the ThinPrep Genesis processor, an instrument that prepares slides for cytology and aliquot samples for molecular testing. Compared to older instruments, the ThinPrep Genesis processor has increased automation capabilities and provides ergonomic and chain-of-custody benefits. It also provides automated sample barcoding, helping to ensure accurate sample tracking and reducing manual steps.
Hologic attributed the lower ThinPrep test volumes in the United States to screening interval expansion and a decline in average selling prices.
The Cytology & Perinatal product lineup was not the only unprofitable portfolio, though. Women’s Health finished fiscal 2019 with a deficit due to lower MonaLisa Touch gynecologic laser sales, which fell victim to an FDA crackdown on “vaginal rejuvenation” devices. These products use lasers and other energy-based devices to remove or reshape vaginal tissue in an effort to treat conditions and symptoms of menopause, urinary incontinence, or sexual function. But clinical evidence on such devices is lacking, and the FDA has identified numerous cases of vaginal burns or scarring tied to vaginal rejuvenation, as well as post-procedural pain during sexual intercourse or recurring chronic pain.
As a result of the FDA’s concerns, Hologic temporarily suspended sales of its TempSure Vitalia handpieces and single-use probes. The products re-entered the market in Q1 of fiscal 2019.
The loss in Women’s Health sales was partly responsible for the FY19 deficit in Medical Aesthetics product revenue. Other contributors to the 9.2 percent sales decrease (to $252.9 million) included lower body contouring product demand; fewer sales of SculpSure lasers, Submental upgrades, and related PAC keys, increased competition in non-invasive fat-reduction technology, and fluctuating foreign currency exchange rates.
Offsetting the Medical Aesthetics division’s profit-busters was higher skin product revenue, driven mainly by robust Icon and TempSure system sales.
Similarly, increased MyoSure and Fluent systems volume helped boost GYN Surgical product revenue 3.6 percent in FY19 to $436.2 million. MyoSure contributed $9.7 million to the total, while Fluent chipped in $10.8 million. Also supporting GYN Surgical’s overall sales increase was the first-quarter U.S. launch of the Omni hysteroscope, a three-in-one modular scope with advanced visualization capabilities designed for both diagnostic and therapeutic procedures. Approved by Canadian and European regulators in March 2019, the Omni scope can be used in doctors’ offices, surgical centers, or operating rooms.
The increases realized through Fluent, MyoSure, and Omni sales were partially neutralized by a $14 million loss in NovaSure systems revenue. Hologic attributed that shortfall to increased competition, a stagnant market for endometrial ablation, and lower average selling prices.
Another profit-maker for Hologic in fiscal 2019 was its Service and Other Revenues division, comprised primarily of sales generated by the firm’s field service organization for ongoing product service, installation, and repair. Most of the proceeds in this division come from the Breast Health segment, and to a lesser extent, the Medical Aesthetics business. Service and Other Revenues rose 3.8 percent to $596 million on higher installation rates, spare parts, and service contract conversion and license renewal rates for the Breast Health business.
Hologic bolstered the Breast Health business in FY19 with its purchase of a 46 percent stake in French firm SuperSonic Imagine. SuperSonic’s products complement Hologic’s wireless, handheld ultrasound scanner Viera, which is commercialized through a development and distribution agreement with Clarius Mobile Health.
Upon purchase of the SuperSonic stake, MacMillan said: “SuperSonic Imagine provides best-in-class ultrasound technology that is strategically important to the long-term growth of our Breast Health business.”
A slew of product launches and approvals throughout FY19 helped foster the growth of Hologic’s other businesses. Those actions included:
- Cynosure’s (which was divested in late 2019) North American launch of TempSure Surgical RF Technology, a new platform offering that allows clinicians to perform both surgical and non-surgical aesthetic procedures across various specialties, on one device.
- The launch of the Unifi Analytics software tool, intended to help healthcare facilities manage mammography usage and performance. The system tracks installed mammography devices and provides efficiency and accuracy analyses for devices and technologists.
- The U.S., Canadian, and European launch of the Trident HD Specimen Radiography System. The product delivers enhanced image quality and improved workflow with instant sample verification during breast-conserving surgeries and stereotactic breast biopsies. The Trident system features a bigger detector that gives clinicians a complete image of larger breast surgical specimens and various surgical and biopsy samples.
- FDA clearance of the LOCalizer radio frequency identification lesion localizing system for tagging and pinpointing breast lesions during surgeries. The LOCalizer does not emit ionizing radiation and can be implanted via syringe up to a month before surgery without anything remaining protruding through the skin.
COVID-19 Consequences
Q2 2020 Revenue: $756 Million
Q2 2019 Revenue: $818 Million
Percentage Change: -7.6%
Two months after securing that authorization, Hologic won a second EUA from the FDA (May 15) for its Aptima SARS-CoV-2 virus detection assay. This test runs on Hologic’s fully automated Panther system, more than 1,000 of which are installed in U.S. clinical laboratories. Each Panther system provides initial results in roughly three hours and can process more than 1,000 COVID-19 tests in 24 hours. More than 1,800 Panther systems have been installed in 60 countries, according to the company.