Business Wire04.30.20
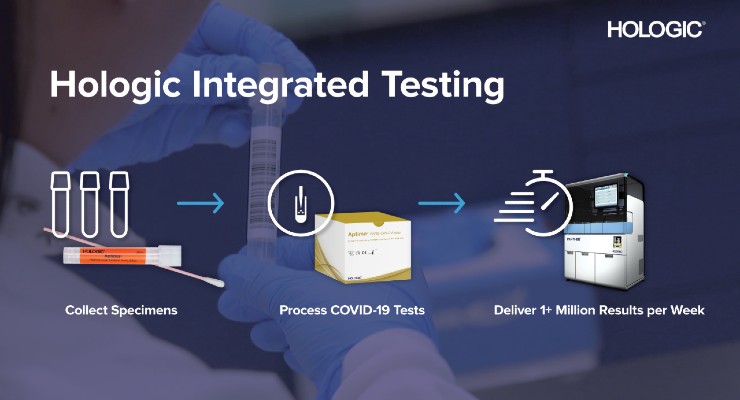
Hologic Inc. plans to launch a new Aptima molecular assay to detect the SARS-CoV-2 virus that will run on its market-leading Panther system. Combining significant manufacturing capacity for the new test with the world’s largest installed base of high-throughput molecular instruments is expected to dramatically increase testing capabilities for the novel coronavirus.
Next week, Hologic expects to begin distributing a Research Use Only (RUO) version of its Aptima SARS-CoV-2 test to hospital, public health and reference laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) to perform high complexity tests. These labs may use the assay for clinical testing on Hologic’s Panther system after completing performance verification testing. Also next week, Hologic plans to apply for Emergency Use Authorization (EUA) for the Aptima SARS-CoV-2 assay from the U.S. Food and Drug Administration. Hologic plans to register a CE mark for diagnostic use in Europe later in May.
Hologic expects to provide its laboratory customers approximately 3 million RUO tests initially. Starting in late May, the company expects to begin producing at least one million Aptima SARS-CoV-2 assays per week on average. Hologic is also planning to increase its production capacity further in the coming months.
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract no. 75A50120P00069.
“Our second COVID-19 test leverages the same proprietary Aptima chemistry and Panther instrumentation that have made Hologic a leader in molecular diagnostics for other infectious diseases,” said Steve MacMillan, the company’s Chairman, president, and CEO. “The ability to deliver test results when and where they are needed—so people can either get back to work or quarantine themselves—has emerged as a key to re-opening global economies. We are responding to this need by developing a second test that can be produced in much larger quantities than our first, and run on a much larger installed base of instruments.”
Curious how medtech's top companies are responding to the COVID-19 pandemic? Get insights into each company's actions by clicking here.
The Aptima SARS-CoV-2 assay runs on the Panther system, a fully automated, high-throughput molecular diagnostic platform that is widely used around the world, with more than 1,800 systems installed in 60 countries. In the United States, just over 1,000 instruments are installed across all 50 states. Each Panther system can provide initial results in approximately three hours and process more than 1,000 coronavirus tests in a 24-hour period. Approximately 750 U.S. hospital, public health and reference labs use the Panther system and its suite of proprietary Aptima technologies—including transcription-mediated amplification (TMA)—to perform tens of millions of molecular tests annually for sexually transmitted infections, cervical cancer screening and viral load monitoring in people with HIV and hepatitis.
In March 2020, Hologic received Emergency Use Authorization for its first COVID-19 test, the Panther Fusion SARS-CoV-2 assay. The Panther Fusion system, an add-on module to the base Panther platform, uses polymerase chain reaction (PCR) chemistry to perform Hologic’s suite of respiratory assays such as influenza. Laboratories can use the Panther Fusion system to test a single patient sample for SARS-CoV-2 and other respiratory viruses that can cause similar symptoms, increasing efficiency and clinical insight. Approximately 200 Panther Fusion systems are installed globally, with roughly half of these in the United States.
“When the COVID-19 pandemic emerged, our initial priority was speed to market, and the Open Access™ software on the Panther Fusion system allowed us to develop a test within a couple of months,” said Kevin Thornal, president of Diagnostic Solutions at Hologic. “Since then, however, testing needs have grown exponentially, both to control the virus and to help unlock economies. We are proud to have marshalled our human and technological resources, and our massive Aptima production capacity, to bring to market a test that can be implemented more broadly.”
Because Hologic’s supply chain has been geared to produce tens of millions of Aptima tests annually for other infectious diseases, the company can redirect these manufacturing resources to produce large quantities of coronavirus assays. In addition, use of Hologic’s Aptima assays do not require additional sample preparation steps or commercial reagents from other vendors, which is expected to help reduce competition for raw materials and increase global testing capacity. Finally, to help alleviate shortages of commonly used sample collection swabs and transport media, Hologic has validated its Aptima Multitest Swab Specimen Collection Kit for testing with both the Aptima and Panther Fusion SARS-CoV-2 assays.
“We have been working closely with government agencies throughout Europe to make plans to provide our Aptima assays to countries across the continent starting in May,” said Jan Verstreken, Hologic’s regional president, EMEA and Canada. “With a broad assay menu already being used in laboratories around the world and Hologic’s ability to make millions of tests over time, we believe the Panther system may provide an ideal solution to help labs meet their testing needs during this critical period, which in turn will help re-open economies safely.”
Hologic’s molecular diagnostic tests are manufactured in the company’s production facilities in San Diego, California and Manchester, United Kingdom. The company is making investments to increase production capacity at both facilities by the fall.
Next week, Hologic expects to begin distributing a Research Use Only (RUO) version of its Aptima SARS-CoV-2 test to hospital, public health and reference laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) to perform high complexity tests. These labs may use the assay for clinical testing on Hologic’s Panther system after completing performance verification testing. Also next week, Hologic plans to apply for Emergency Use Authorization (EUA) for the Aptima SARS-CoV-2 assay from the U.S. Food and Drug Administration. Hologic plans to register a CE mark for diagnostic use in Europe later in May.
Hologic expects to provide its laboratory customers approximately 3 million RUO tests initially. Starting in late May, the company expects to begin producing at least one million Aptima SARS-CoV-2 assays per week on average. Hologic is also planning to increase its production capacity further in the coming months.
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract no. 75A50120P00069.
“Our second COVID-19 test leverages the same proprietary Aptima chemistry and Panther instrumentation that have made Hologic a leader in molecular diagnostics for other infectious diseases,” said Steve MacMillan, the company’s Chairman, president, and CEO. “The ability to deliver test results when and where they are needed—so people can either get back to work or quarantine themselves—has emerged as a key to re-opening global economies. We are responding to this need by developing a second test that can be produced in much larger quantities than our first, and run on a much larger installed base of instruments.”
Curious how medtech's top companies are responding to the COVID-19 pandemic? Get insights into each company's actions by clicking here.
The Aptima SARS-CoV-2 assay runs on the Panther system, a fully automated, high-throughput molecular diagnostic platform that is widely used around the world, with more than 1,800 systems installed in 60 countries. In the United States, just over 1,000 instruments are installed across all 50 states. Each Panther system can provide initial results in approximately three hours and process more than 1,000 coronavirus tests in a 24-hour period. Approximately 750 U.S. hospital, public health and reference labs use the Panther system and its suite of proprietary Aptima technologies—including transcription-mediated amplification (TMA)—to perform tens of millions of molecular tests annually for sexually transmitted infections, cervical cancer screening and viral load monitoring in people with HIV and hepatitis.
In March 2020, Hologic received Emergency Use Authorization for its first COVID-19 test, the Panther Fusion SARS-CoV-2 assay. The Panther Fusion system, an add-on module to the base Panther platform, uses polymerase chain reaction (PCR) chemistry to perform Hologic’s suite of respiratory assays such as influenza. Laboratories can use the Panther Fusion system to test a single patient sample for SARS-CoV-2 and other respiratory viruses that can cause similar symptoms, increasing efficiency and clinical insight. Approximately 200 Panther Fusion systems are installed globally, with roughly half of these in the United States.
“When the COVID-19 pandemic emerged, our initial priority was speed to market, and the Open Access™ software on the Panther Fusion system allowed us to develop a test within a couple of months,” said Kevin Thornal, president of Diagnostic Solutions at Hologic. “Since then, however, testing needs have grown exponentially, both to control the virus and to help unlock economies. We are proud to have marshalled our human and technological resources, and our massive Aptima production capacity, to bring to market a test that can be implemented more broadly.”
Because Hologic’s supply chain has been geared to produce tens of millions of Aptima tests annually for other infectious diseases, the company can redirect these manufacturing resources to produce large quantities of coronavirus assays. In addition, use of Hologic’s Aptima assays do not require additional sample preparation steps or commercial reagents from other vendors, which is expected to help reduce competition for raw materials and increase global testing capacity. Finally, to help alleviate shortages of commonly used sample collection swabs and transport media, Hologic has validated its Aptima Multitest Swab Specimen Collection Kit for testing with both the Aptima and Panther Fusion SARS-CoV-2 assays.
“We have been working closely with government agencies throughout Europe to make plans to provide our Aptima assays to countries across the continent starting in May,” said Jan Verstreken, Hologic’s regional president, EMEA and Canada. “With a broad assay menu already being used in laboratories around the world and Hologic’s ability to make millions of tests over time, we believe the Panther system may provide an ideal solution to help labs meet their testing needs during this critical period, which in turn will help re-open economies safely.”
Hologic’s molecular diagnostic tests are manufactured in the company’s production facilities in San Diego, California and Manchester, United Kingdom. The company is making investments to increase production capacity at both facilities by the fall.