07.19.16
$2.4 Billion
KEY EXECUTIVES:
Gary S. Guthart, Ph.D., President and CEO
Dave J. Rosa, Exec. VP and Chief Commercial Officer
Salvatore J. Brogna, Exec. VP, Product Operations
Myriam J. Curet, M.D., Sr. VP, Chief Medical Officer
Marshall L. Mohr, Sr. VP and Chief Financial Officer
Bob DeSantis, Sr. VP, Instruments and Accessories
Mark Johnson, Sr. VP, Regulatory and Quality
Charles Jones, Sr. VP, Design and User Experience
Brian Miller, Ph.D., Sr. VP, Product Development
NUMBER OF EMPLOYEES: 3,211
GLOBAL HEADQUARTERS: Sunnyvale, Calif.
The plethora of predictions about professionals being replaced by robotic substitutes reached an apex in 2015, as analyst after analyst published projections of likely candidates for bionic supplantation. (Go on, google “will a robot take my job” and see how many different results come up.)
Unless robotic synaptic technology experiences significant growth soon, journalists should have nothing to fear—it was mostly manufacturing employees projected to be replaced, as automation robots designed for routine manufacturing processes became standard practice in the plant. But surely a robot couldn’t replace a doctor: Metal hands are far too clumsy to handle the intricacies of human surgery, right?
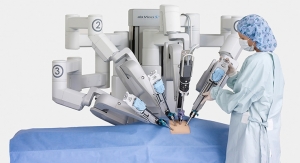
Intuitive Surgical Inc.’s da Vinci surgical system, which first received U.S. Food and Drug Administration (FDA) clearance in 2000, indicates otherwise. Surgeons need not worry too much about being replaced; da Vinci still requires a surgeon at the helm in order to perform the procedure. Although it can be used for a slew of minimally invasive procedures (which will not be listed lest this entry become egregiously long; take a look at Intuitive’s website to see just how much this robot can do), it still lacks the decision-making software to make it fully autonomous...for now.
Financial Landscape
Intuitive Surgical’s product portfolio revolves almost entirely around its da Vinci system. (The company also provides the InSite and Firely Fluorescence imaging systems as peripherals to da Vinci.) The company’s business segments are broken up into categories based on the instruments and accessories for the da Vinci system, sales of the systems, and services such as installation and training to use the system. Some worry that the company is essentially putting all its eggs into one basket, leaving limited room for growth. However, those eggs can perform an astonishing (and increasing) variety of minimally invasive surgeries—which should alleviate some of that anxiety.
A particularly good sign is the fact that Intuitive Surgical made its debut in this year’s Top 30 listing. Intuitive’s fiscal 2015 (year ended Dec. 31) netted the firm $2.38 billion in sales, a 12 percent increase from 2014. This is a fairly significant rebound from 2014’s deficit (down 5 percent from 2013), which was due primarily to 24 percent lower sales of the da Vinci system, mainly in the United States and Japan. In 2015, 71 percent of revenue consisted of domestic net sales, and 29 percent comprised what the company calls “OUS” or outside the United States. This ratio has remained relatively stable through the last few years, with 70 percent and 30 percent in 2014 and
72 percent and 28 percent in 2013, respectively.
Intuitive’s phased global launch of the da Vinci Xi system in mid-2014 certainly had an effect on product net sales. In 2015, product revenue amounted to $1.9 billion, a 13 percent increase over 2014, largely due to the system’s launch. Systems revenue overall was $721.9 million, a 14 percent increase over 2014. Due to a higher product mix of advanced instruments (though offset somewhat by weakening foreign currencies), instruments and accessories revenue for fiscal year 2015 totaled $1.2 billion, a 12 percent increase over 2014. Contributing to this increase was a 14 percent higher procedure volume, with 11 percent domestic growth and 26 percent OUS growth, respectively. The larger sales volume of da Vinci systems worldwide also contributed to service revenue of $464.8 million, representing an 8 percent increase over 2014.
What’s New with da Vinci?
Intuitive’s latest iteration of its flagship product, the da Vinci Xi, launched in the United States in April 2014. As mentioned previously, the favorable market response to the new system was a large driver in the company’s overall revenue increase: 492 da Vinci systems were sold globally in 2015, 61 more than the year prior. As a result, da Vinci procedures also experienced a rise: in fiscal 2015, approximately 652,000 procedures were performed, about a 14 percent increase from fiscal 2014.
da Vinci Xi continues to build on the core systems features. It now includes wristed instruments, 3D high-definition visualization, intuitive motion, and an ergonomic design. Specifically, the newest version contains an overhead instrument arm to facilitate anatomical access from basically any position and a simpler, more compact endoscope digital architecture with improved vision definition and clarity (which can be attached to any arm). The arms themselves were designed to be smaller and thinner with joints that offer a more flexible range of motion, and the system’s longer instrument shafts offer surgeons greater operative reach. Xi was CE marked in June 2014, cleared in South Korea in October 2014, and cleared in Japan in March 2015. da Vinci Xi versions of the EndoWrist stapler 45—a wristed, stapling instrument meant for resection, transection, and/or anastomoses creation in general, gynecologic, and urologic surgery—were initially shipped in January 2015. The EndoWrist Stapler was CE marked to sell in European markets for the Si and Xi surgical systems in April 2015.
CE mark clearance was also achieved for da Vinci Xi Integrated Table Motion product in June 2015. This coordinates da Vinci’s robot arms with a version of the Trumpf Medical TruSystem 7000dV operating room table, enabling patient position shifting in real-time while the arms remain docked. It permits OR teams to optimally position the operating table so gravity exposes anatomy during multi-quadrant procedures, allows surgeons to interact with tissues at an ideal working angle, and repositions the table during procedures to assist the anesthesiologists’ care. It was introduced in phases to the European market in fourth quarter 2015, and was cleared by the FDA in January 2016.
With Great Innovation Comes Great Litigation
As of Dec. 31, 2015, Intuitive was named as a defendant in about 92 individual product liability lawsuits, alleging that a variety of personal injuries—and in some cases, death—resulted from botched da Vinci surgical system procedures. In fact, as recently as Dec. 21, plaintiffs in a Missouri legal action added 10 additional plaintiffs, seeking damages on behalf of 55 patients who underwent da Vinci surgeries in 22 different states. This isn’t exactly news for Intuitive, which has been receiving a steady stream of lawsuits related to da Vinci procedures gone awry since the product’s launch. The most recent allegations assert that da Vinci’s defects and/or Intuitive’s failure to adequately train healthcare professionals performing the surgeries resulted in the injuries. Further, the plaintiffs claim that Intuitive did not disclose and/or misrepresented the benefits and risks of the device.
Initiated by plaintiffs’ attorneys, da Vinci has also been subject to well-funded national advertising efforts directed at patients dissatisfied with their surgery. The company has received a significant amount of claims relating to alleged surgical complications from surgeries using the Monopolar Curved Scissor (MCS) instruments—the tip cover accessory for these were withdrawn from the market in 2012, and MCS instruments were the subject of a recall in 2013. One recent case, which is now before the Washington State Supreme Court awaiting review, specifically accused the company of failing to properly train, warn, and instruct the operating surgeon.
Product liability litigation is such a concern for Intuitive that the company often enters into tolling agreements to minimize the expense and distraction of defending multiple lawsuits. Pre-tax charges of these agreements were $13.8 million, thankfully significantly down from 2014’s $82.4 million.
Research and Training Initiatives
Toward the end of February 2015, Intuitive began a partnership with the American Hernia Society Quality Collaborative (AHSQC). Joining six other foundation partners, this initiated a surgeon-led, online quality initiative to share real-time patient data regarding abdominal hernias, review peer and institutional data, and analyze best practices, decision support, and care pathways. “It is critical that we follow and track the results of our operations to determine the best options that help our patients. We should all take responsibility for fostering a culture of quality improvement,” Dr. Benjamin K. Poulose, M.P.H., AHSQC director of quality and outcomes, said in a company press release. The study sought to address and improve upon health outcomes for hernia patients as well as optimize costs for surgical hernia repair by assessing factors responsible for hernia occurrence, quality of life following hernia repair, reducing surgical-site complications, evaluating advantages of minimally invasive repair, and minimizing perioperative pain.
The company announced a different type of quality initiative in August 2015, when simulator grants were awarded by Intuitive to five top U.S. medical centers to advance training in robotic-assisted minimally invasive surgery. The goal was to interpret how surgical skills learned in a virtual-reality environment translate to improved inter-operative skills, simultaneously identifying training needs and determining the research required to address those needs.
“As the da Vinci Surgical Systems emerge on the forefront of minimally invasive surgery, validation of safe training curricula for residents, fellows, and practicing surgeons will have to be sought with careful, scientific rigor,” Shawn Tsuda, M.D., FACS, associate professor of surgery at the University of Nevada School of Medicine, said in a press release. “This grant enables forward-looking programs to develop best-practices for integrating robotics into surgical training.”
KEY EXECUTIVES:
Gary S. Guthart, Ph.D., President and CEO
Dave J. Rosa, Exec. VP and Chief Commercial Officer
Salvatore J. Brogna, Exec. VP, Product Operations
Myriam J. Curet, M.D., Sr. VP, Chief Medical Officer
Marshall L. Mohr, Sr. VP and Chief Financial Officer
Bob DeSantis, Sr. VP, Instruments and Accessories
Mark Johnson, Sr. VP, Regulatory and Quality
Charles Jones, Sr. VP, Design and User Experience
Brian Miller, Ph.D., Sr. VP, Product Development
NUMBER OF EMPLOYEES: 3,211
GLOBAL HEADQUARTERS: Sunnyvale, Calif.
The plethora of predictions about professionals being replaced by robotic substitutes reached an apex in 2015, as analyst after analyst published projections of likely candidates for bionic supplantation. (Go on, google “will a robot take my job” and see how many different results come up.)
Unless robotic synaptic technology experiences significant growth soon, journalists should have nothing to fear—it was mostly manufacturing employees projected to be replaced, as automation robots designed for routine manufacturing processes became standard practice in the plant. But surely a robot couldn’t replace a doctor: Metal hands are far too clumsy to handle the intricacies of human surgery, right?
Intuitive Surgical Inc.’s da Vinci surgical system, which first received U.S. Food and Drug Administration (FDA) clearance in 2000, indicates otherwise. Surgeons need not worry too much about being replaced; da Vinci still requires a surgeon at the helm in order to perform the procedure. Although it can be used for a slew of minimally invasive procedures (which will not be listed lest this entry become egregiously long; take a look at Intuitive’s website to see just how much this robot can do), it still lacks the decision-making software to make it fully autonomous...for now.
Financial Landscape
Intuitive Surgical’s product portfolio revolves almost entirely around its da Vinci system. (The company also provides the InSite and Firely Fluorescence imaging systems as peripherals to da Vinci.) The company’s business segments are broken up into categories based on the instruments and accessories for the da Vinci system, sales of the systems, and services such as installation and training to use the system. Some worry that the company is essentially putting all its eggs into one basket, leaving limited room for growth. However, those eggs can perform an astonishing (and increasing) variety of minimally invasive surgeries—which should alleviate some of that anxiety.
A particularly good sign is the fact that Intuitive Surgical made its debut in this year’s Top 30 listing. Intuitive’s fiscal 2015 (year ended Dec. 31) netted the firm $2.38 billion in sales, a 12 percent increase from 2014. This is a fairly significant rebound from 2014’s deficit (down 5 percent from 2013), which was due primarily to 24 percent lower sales of the da Vinci system, mainly in the United States and Japan. In 2015, 71 percent of revenue consisted of domestic net sales, and 29 percent comprised what the company calls “OUS” or outside the United States. This ratio has remained relatively stable through the last few years, with 70 percent and 30 percent in 2014 and
72 percent and 28 percent in 2013, respectively.
Intuitive’s phased global launch of the da Vinci Xi system in mid-2014 certainly had an effect on product net sales. In 2015, product revenue amounted to $1.9 billion, a 13 percent increase over 2014, largely due to the system’s launch. Systems revenue overall was $721.9 million, a 14 percent increase over 2014. Due to a higher product mix of advanced instruments (though offset somewhat by weakening foreign currencies), instruments and accessories revenue for fiscal year 2015 totaled $1.2 billion, a 12 percent increase over 2014. Contributing to this increase was a 14 percent higher procedure volume, with 11 percent domestic growth and 26 percent OUS growth, respectively. The larger sales volume of da Vinci systems worldwide also contributed to service revenue of $464.8 million, representing an 8 percent increase over 2014.
What’s New with da Vinci?
Intuitive’s latest iteration of its flagship product, the da Vinci Xi, launched in the United States in April 2014. As mentioned previously, the favorable market response to the new system was a large driver in the company’s overall revenue increase: 492 da Vinci systems were sold globally in 2015, 61 more than the year prior. As a result, da Vinci procedures also experienced a rise: in fiscal 2015, approximately 652,000 procedures were performed, about a 14 percent increase from fiscal 2014.
da Vinci Xi continues to build on the core systems features. It now includes wristed instruments, 3D high-definition visualization, intuitive motion, and an ergonomic design. Specifically, the newest version contains an overhead instrument arm to facilitate anatomical access from basically any position and a simpler, more compact endoscope digital architecture with improved vision definition and clarity (which can be attached to any arm). The arms themselves were designed to be smaller and thinner with joints that offer a more flexible range of motion, and the system’s longer instrument shafts offer surgeons greater operative reach. Xi was CE marked in June 2014, cleared in South Korea in October 2014, and cleared in Japan in March 2015. da Vinci Xi versions of the EndoWrist stapler 45—a wristed, stapling instrument meant for resection, transection, and/or anastomoses creation in general, gynecologic, and urologic surgery—were initially shipped in January 2015. The EndoWrist Stapler was CE marked to sell in European markets for the Si and Xi surgical systems in April 2015.
CE mark clearance was also achieved for da Vinci Xi Integrated Table Motion product in June 2015. This coordinates da Vinci’s robot arms with a version of the Trumpf Medical TruSystem 7000dV operating room table, enabling patient position shifting in real-time while the arms remain docked. It permits OR teams to optimally position the operating table so gravity exposes anatomy during multi-quadrant procedures, allows surgeons to interact with tissues at an ideal working angle, and repositions the table during procedures to assist the anesthesiologists’ care. It was introduced in phases to the European market in fourth quarter 2015, and was cleared by the FDA in January 2016.
With Great Innovation Comes Great Litigation
As of Dec. 31, 2015, Intuitive was named as a defendant in about 92 individual product liability lawsuits, alleging that a variety of personal injuries—and in some cases, death—resulted from botched da Vinci surgical system procedures. In fact, as recently as Dec. 21, plaintiffs in a Missouri legal action added 10 additional plaintiffs, seeking damages on behalf of 55 patients who underwent da Vinci surgeries in 22 different states. This isn’t exactly news for Intuitive, which has been receiving a steady stream of lawsuits related to da Vinci procedures gone awry since the product’s launch. The most recent allegations assert that da Vinci’s defects and/or Intuitive’s failure to adequately train healthcare professionals performing the surgeries resulted in the injuries. Further, the plaintiffs claim that Intuitive did not disclose and/or misrepresented the benefits and risks of the device.
Initiated by plaintiffs’ attorneys, da Vinci has also been subject to well-funded national advertising efforts directed at patients dissatisfied with their surgery. The company has received a significant amount of claims relating to alleged surgical complications from surgeries using the Monopolar Curved Scissor (MCS) instruments—the tip cover accessory for these were withdrawn from the market in 2012, and MCS instruments were the subject of a recall in 2013. One recent case, which is now before the Washington State Supreme Court awaiting review, specifically accused the company of failing to properly train, warn, and instruct the operating surgeon.
Product liability litigation is such a concern for Intuitive that the company often enters into tolling agreements to minimize the expense and distraction of defending multiple lawsuits. Pre-tax charges of these agreements were $13.8 million, thankfully significantly down from 2014’s $82.4 million.
Research and Training Initiatives
Toward the end of February 2015, Intuitive began a partnership with the American Hernia Society Quality Collaborative (AHSQC). Joining six other foundation partners, this initiated a surgeon-led, online quality initiative to share real-time patient data regarding abdominal hernias, review peer and institutional data, and analyze best practices, decision support, and care pathways. “It is critical that we follow and track the results of our operations to determine the best options that help our patients. We should all take responsibility for fostering a culture of quality improvement,” Dr. Benjamin K. Poulose, M.P.H., AHSQC director of quality and outcomes, said in a company press release. The study sought to address and improve upon health outcomes for hernia patients as well as optimize costs for surgical hernia repair by assessing factors responsible for hernia occurrence, quality of life following hernia repair, reducing surgical-site complications, evaluating advantages of minimally invasive repair, and minimizing perioperative pain.
The company announced a different type of quality initiative in August 2015, when simulator grants were awarded by Intuitive to five top U.S. medical centers to advance training in robotic-assisted minimally invasive surgery. The goal was to interpret how surgical skills learned in a virtual-reality environment translate to improved inter-operative skills, simultaneously identifying training needs and determining the research required to address those needs.
“As the da Vinci Surgical Systems emerge on the forefront of minimally invasive surgery, validation of safe training curricula for residents, fellows, and practicing surgeons will have to be sought with careful, scientific rigor,” Shawn Tsuda, M.D., FACS, associate professor of surgery at the University of Nevada School of Medicine, said in a press release. “This grant enables forward-looking programs to develop best-practices for integrating robotics into surgical training.”