PR Newswire05.23.17
EnteroMedics Inc. the developer of medical devices treating obesity, metabolic diseases, and other gastrointestinal disorders, announced that it has acquired the Gastric Vest System through its acquisition of BarioSurg Inc.
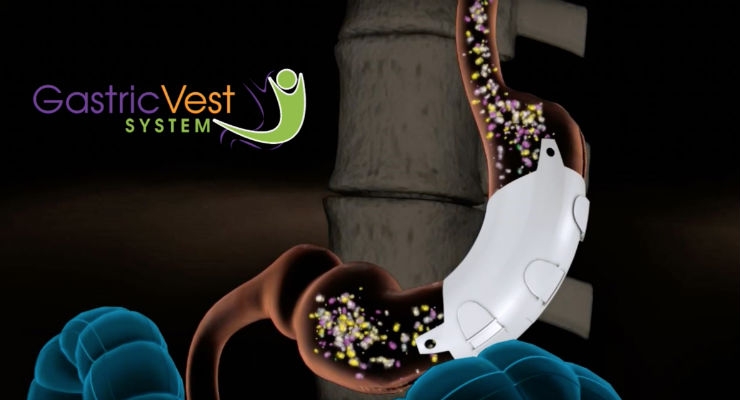
The Gastric Vest is an investigational, minimally-invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients. The device, which wraps around the stomach and emulates the effect of conventional weight loss surgery, enables gastric volume reduction without permanently changing patient anatomy. In a pilot study conducted outside the U.S., at 12 months, Vest patients demonstrated a mean percent excess weight loss (%EWL) of 85 percent, an average drop in HbA1c (Hemoglobin A1c) of 2.1 points, and an average waist circumference reduction of 38 centimeters, or approximately 15 inches.
"We are excited to both incorporate BarioSurg's Gastric Vest into our now further differentiated portfolio of medical devices for fighting obesity and related comorbidities, and to explore potential clinical opportunities to combine the Vest and vBloc Therapy," said Dan Gladney, EnteroMedics president, chief executive officer, and chairman of the board. "As we move toward building a comprehensive bariatric and metabolic continuum of care platform to effectively address unmet needs within these areas, we believe that the strong foundation we've built at EnteroMedics will maximize the potential for a successful approval and the subsequent commercialization of this device. We look forward to sharing more details on this morning's conference call."
"Based on early results, when comparing short-term %EWL, the Gastric Vest appears to perform as well as, and possibly even better than, gastric bypass and sleeve gastrectomy procedures," stated Scott Shikora, MD, Director, Center for Metabolic and Bariatric Surgery, Brigham and Women's Hospital, former president of the American Society for Metabolic and Bariatric Surgery, and chief medical consultant, EnteroMedics. "If the Vest continues to yield similar results to those observed to date, it will be a game changer in the field of bariatrics."
Raj Nihalani, M.D., inventor of the Gastric Vest System, founder and former chief executive officer of BarioSurg stated: "EnteroMedics is a leader in minimally-invasive, sustainable weight loss treatment with vBloc Therapy. I look forward to joining the company and navigating a path toward potential FDA approval and eventual commercialization, while at the same time exploring ways in which the Vest may be able to be combined with vBloc Therapy to enhance patient outcomes."
The consideration paid by EnteroMedics for BarioSurg Inc. consists of 1.38 million unregistered shares of EnteroMedics common stock, 1.0 million unregistered shares of conditional convertible preferred stock (which will be convertible into 5.0 million unregistered shares of common stock upon the receipt of the required approval of EnteroMedics' stockholders under NASDAQ rules), and $2.0 million in cash. The shares of common stock issued in the acquisition represent 19.99% of EnteroMedics' outstanding common stock immediately prior to the acquisition. EnteroMedics expects to hold a special meeting of its stockholders to seek the required approval of the conversion of the conditional convertible preferred stock in the summer of 2017.
In connection with the acquisition, EnteroMedics has appointed Dr. Nihalani as chief technology officer, EnteroMedics.
The Gastric Vest is an investigational, minimally-invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients. The device, which wraps around the stomach and emulates the effect of conventional weight loss surgery, enables gastric volume reduction without permanently changing patient anatomy. In a pilot study conducted outside the U.S., at 12 months, Vest patients demonstrated a mean percent excess weight loss (%EWL) of 85 percent, an average drop in HbA1c (Hemoglobin A1c) of 2.1 points, and an average waist circumference reduction of 38 centimeters, or approximately 15 inches.
"We are excited to both incorporate BarioSurg's Gastric Vest into our now further differentiated portfolio of medical devices for fighting obesity and related comorbidities, and to explore potential clinical opportunities to combine the Vest and vBloc Therapy," said Dan Gladney, EnteroMedics president, chief executive officer, and chairman of the board. "As we move toward building a comprehensive bariatric and metabolic continuum of care platform to effectively address unmet needs within these areas, we believe that the strong foundation we've built at EnteroMedics will maximize the potential for a successful approval and the subsequent commercialization of this device. We look forward to sharing more details on this morning's conference call."
"Based on early results, when comparing short-term %EWL, the Gastric Vest appears to perform as well as, and possibly even better than, gastric bypass and sleeve gastrectomy procedures," stated Scott Shikora, MD, Director, Center for Metabolic and Bariatric Surgery, Brigham and Women's Hospital, former president of the American Society for Metabolic and Bariatric Surgery, and chief medical consultant, EnteroMedics. "If the Vest continues to yield similar results to those observed to date, it will be a game changer in the field of bariatrics."
Raj Nihalani, M.D., inventor of the Gastric Vest System, founder and former chief executive officer of BarioSurg stated: "EnteroMedics is a leader in minimally-invasive, sustainable weight loss treatment with vBloc Therapy. I look forward to joining the company and navigating a path toward potential FDA approval and eventual commercialization, while at the same time exploring ways in which the Vest may be able to be combined with vBloc Therapy to enhance patient outcomes."
The consideration paid by EnteroMedics for BarioSurg Inc. consists of 1.38 million unregistered shares of EnteroMedics common stock, 1.0 million unregistered shares of conditional convertible preferred stock (which will be convertible into 5.0 million unregistered shares of common stock upon the receipt of the required approval of EnteroMedics' stockholders under NASDAQ rules), and $2.0 million in cash. The shares of common stock issued in the acquisition represent 19.99% of EnteroMedics' outstanding common stock immediately prior to the acquisition. EnteroMedics expects to hold a special meeting of its stockholders to seek the required approval of the conversion of the conditional convertible preferred stock in the summer of 2017.
In connection with the acquisition, EnteroMedics has appointed Dr. Nihalani as chief technology officer, EnteroMedics.