Business Wire04.12.17
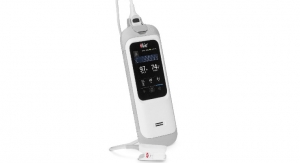
Ventec Life Systems, a developer of integrated respiratory care solutions, announced it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for VOCSN, the only portable life support device to combine five respiratory therapies —Ventilation, Oxygen, Cough, Suction, and Nebulization (VOCSN, pronounced VOX-SEN).
VOCSN works across the continuum of care, from the hospital to home, and for pediatric patients weighing more than 5 kg to adults. VOCSN is designed to improve care for patients with neuromuscular disease (e.g., Muscular Dystrophies, ALS), impaired lung function (e.g., COPD, Cystic Fibrosis, Lung Cancers, Emphysema), spinal cord injury, and pediatric development complication (e.g., premature births, Chronic Lung Disease). Patients can get all five therapies or just the mix of therapies needed.
VOCSN is more than 70% lighter and smaller than existing machines, features a nine-hour on-board battery, and is controlled through an intuitive touchscreen interface and user-friendly operating system. With VOCSN, caregivers can seamlessly switch between therapies with the touch of a button and no longer need to change the patient circuit between therapies. VOCSN enables caregivers to spend less time managing machines and more time caring for patients.
Following his father’s diagnosis with ALS, Doug DeVries, founder and CEO at Ventec, dedicated his work to creating better solutions for ventilator patients, including the portable LTV Series of ventilators. “I’ve seen firsthand how improved ventilator technology can enhance the quality of life for patients and caregivers. Our team didn’t want to create just another ventilator, we spent the past five years focused on building a truly integrated solution.” New technology was developed to provide best-in-class treatment in a smaller, more energy efficient system. VOCSN is engineered and manufactured in the United States with eight pending patents.
VOCSN is inspired by ideas from patients, caregivers, medical professionals, and a team of more than 30 leading engineers. “Since the iron lung, respiratory equipment has limited the mobility of ventilator patients. VOCSN was designed for everyday mobility so that patients can focus on their relationships with loved ones and live their life,” said Richard Branson, Professor of Surgery, University of Cincinnati and a member of the Ventec Clinical Board.
VOCSN is a portable critical care ventilator that provides invasive, noninvasive, and mouthpiece ventilation. Designed to work in hospital, institutional, transport, and home environments, VOCSN delivers a comprehensive set of ventilation modes and settings to meet patient needs. The advanced ventilation technology combines responsive leak and circuit compensation as well as precision flow trigger controls to enable comfortable breathing and accurate therapy.
VOCSN is a portable oxygen concentrator that delivers the equivalent of 6 L/min of oxygen. The VOCSN Oxygen-Direct system is up to three times more energy efficient than existing concentrators. In addition to the on-board internal Oxygen Concentrator, external oxygen sources can be connected to VOCSN for critical care patients. VOCSN also includes an onboard FiO2 monitor to verify accurate oxygen delivery.
By unifying ventilation, cough, and suction into one system, it now takes seconds instead of minutes to administer cough therapy with Touch Button Cough. The Ventec One-Circuit features a high flow valve design, allowing patients to use the same circuit for ventilation and cough. Using the Cough + Suction feature and Ventec Secretion Trap, suction therapy is activated during the cough therapy to clear secretions. Once the set number of cough cycles is complete, ventilation automatically resumes.
The VOCSN high flow suction provides quiet and effective airway clearance with consistent and precise vacuum pressure. The suction system, together with the Ventec Secretion Trap, simplifies mucus management by allowing patients to remain connected to the same circuit during ventilation, cough, and suction therapies. The advanced sound muffler in VOCSN makes suction therapy more than three times quieter than traditional suction machines.
VOCSN automatically compensates for the airflow from the ventilator when the nebulizer drive is active to ensure accurate ventilation. VOCSN records data about each treatment and automatically turns off the nebulizer once the therapy timer is complete.
The Ventec One-Circuit allows switching between therapies with the touch of a button. The innovative circuit design includes four pending patents that make Touch Button Cough™ and the Oxygen Direct™ system possible. Rather than several cumbersome tubes connecting to separate devices, the Ventec One-Circuit eliminates clutter to create one easy-to-manage system for peace of mind.
The Ventec team will continue collaborating with respiratory thought leaders to introduce VOCSN across the continuum of care. Over the next year, Ventec will work with select partners on a controlled rollout to maintain a close connection between patients and caregivers. Limited quantities of VOCSN will ship in the United States beginning in June 2017, Asia in 2017, and Canada and Europe in 2018. VOCSN is customizable to each patient and its transparent pricing model will allow therapies to be priced independently. Patients can get all five therapies or just the mix of therapies needed.
VOCSN works across the continuum of care, from the hospital to home, and for pediatric patients weighing more than 5 kg to adults. VOCSN is designed to improve care for patients with neuromuscular disease (e.g., Muscular Dystrophies, ALS), impaired lung function (e.g., COPD, Cystic Fibrosis, Lung Cancers, Emphysema), spinal cord injury, and pediatric development complication (e.g., premature births, Chronic Lung Disease). Patients can get all five therapies or just the mix of therapies needed.
VOCSN is more than 70% lighter and smaller than existing machines, features a nine-hour on-board battery, and is controlled through an intuitive touchscreen interface and user-friendly operating system. With VOCSN, caregivers can seamlessly switch between therapies with the touch of a button and no longer need to change the patient circuit between therapies. VOCSN enables caregivers to spend less time managing machines and more time caring for patients.
Following his father’s diagnosis with ALS, Doug DeVries, founder and CEO at Ventec, dedicated his work to creating better solutions for ventilator patients, including the portable LTV Series of ventilators. “I’ve seen firsthand how improved ventilator technology can enhance the quality of life for patients and caregivers. Our team didn’t want to create just another ventilator, we spent the past five years focused on building a truly integrated solution.” New technology was developed to provide best-in-class treatment in a smaller, more energy efficient system. VOCSN is engineered and manufactured in the United States with eight pending patents.
VOCSN is inspired by ideas from patients, caregivers, medical professionals, and a team of more than 30 leading engineers. “Since the iron lung, respiratory equipment has limited the mobility of ventilator patients. VOCSN was designed for everyday mobility so that patients can focus on their relationships with loved ones and live their life,” said Richard Branson, Professor of Surgery, University of Cincinnati and a member of the Ventec Clinical Board.
VOCSN is a portable critical care ventilator that provides invasive, noninvasive, and mouthpiece ventilation. Designed to work in hospital, institutional, transport, and home environments, VOCSN delivers a comprehensive set of ventilation modes and settings to meet patient needs. The advanced ventilation technology combines responsive leak and circuit compensation as well as precision flow trigger controls to enable comfortable breathing and accurate therapy.
VOCSN is a portable oxygen concentrator that delivers the equivalent of 6 L/min of oxygen. The VOCSN Oxygen-Direct system is up to three times more energy efficient than existing concentrators. In addition to the on-board internal Oxygen Concentrator, external oxygen sources can be connected to VOCSN for critical care patients. VOCSN also includes an onboard FiO2 monitor to verify accurate oxygen delivery.
By unifying ventilation, cough, and suction into one system, it now takes seconds instead of minutes to administer cough therapy with Touch Button Cough. The Ventec One-Circuit features a high flow valve design, allowing patients to use the same circuit for ventilation and cough. Using the Cough + Suction feature and Ventec Secretion Trap, suction therapy is activated during the cough therapy to clear secretions. Once the set number of cough cycles is complete, ventilation automatically resumes.
The VOCSN high flow suction provides quiet and effective airway clearance with consistent and precise vacuum pressure. The suction system, together with the Ventec Secretion Trap, simplifies mucus management by allowing patients to remain connected to the same circuit during ventilation, cough, and suction therapies. The advanced sound muffler in VOCSN makes suction therapy more than three times quieter than traditional suction machines.
VOCSN automatically compensates for the airflow from the ventilator when the nebulizer drive is active to ensure accurate ventilation. VOCSN records data about each treatment and automatically turns off the nebulizer once the therapy timer is complete.
The Ventec One-Circuit allows switching between therapies with the touch of a button. The innovative circuit design includes four pending patents that make Touch Button Cough™ and the Oxygen Direct™ system possible. Rather than several cumbersome tubes connecting to separate devices, the Ventec One-Circuit eliminates clutter to create one easy-to-manage system for peace of mind.
The Ventec team will continue collaborating with respiratory thought leaders to introduce VOCSN across the continuum of care. Over the next year, Ventec will work with select partners on a controlled rollout to maintain a close connection between patients and caregivers. Limited quantities of VOCSN will ship in the United States beginning in June 2017, Asia in 2017, and Canada and Europe in 2018. VOCSN is customizable to each patient and its transparent pricing model will allow therapies to be priced independently. Patients can get all five therapies or just the mix of therapies needed.