Sunil Jha, Research Content Developer, Global Market Insights02.21.20
With millions of people suffering from heart complications, poor heart pumping has become the major cause of leg swelling, fatigue, difficulty in breathing, and trouble exercising. Even though heart surgery, medication, and lifestyle changes can help alleviate symptoms, patients warrant more help in the form of pacemakers to help their hearts function properly.
Pacemakers have become second to none when it comes to controlling the heartbeat. In common parlance, pacemakers tend to control abnormal heart rhythms and enable the heart to beat at a normal rate using electrical pulses. Notably, pacemakers can rev up a slow heart rhythm, control a fast heart rhythm, and coordinate the chambers of the heart.
Prevalence of arrhythmias has further signified the importance of pacemakers that use low-energy electrical pulses to surmount the faulty electrical signaling. Accordingly, pacemakers can not only control fast or abnormal rhythm, but they also ensure the ventricles contract normally if the atria are quivering and coordinate electrical signaling between the ventricles. Pacemaker devices are also believed to coordinate electrical signaling between the ventricles with the help of cardiac resynchronization therapy (CRT) devices.
Pacemakers Are Here to Stay: MRI Pacemakers Take the World by Storm
The need for pacemakers arises from shortness of breath or that arrhythmias such as tachyarrhythmia and bradyarrhythmia have chances of damaging vital organs. While permanent pacemakers have become the go-to solution, temporary pacemakers are gradually grabbing eyeballs. With an upsurge in the number of people suffering from heart attacks, traction for temporary pacemakers is likely to remain intact in the future.
Reportedly, 1.25 million permanent pacemakers are implanted globally, while around half a million permanent pacemakers were said to be implanted in Europe alone. Temporary pacemakers are used given that the arrhythmias are anticipated to be temporary. Meanwhile, permanent pacemakers find a place for patients with symptomatic bradyarrhythmia or to help kill or prevent tachyarrhythmia.
Technological developments have translated into miniaturization of electronic circuitry and the development of implantable devices. Advances such as device size reduction, internet-based remote monitoring, rate responsiveness, and a notable rise in battery longevity have been linked to implantable pacemakers.
The advent of FDA-cleared MRI pacemakers has taken the world by storm, as these devices have allowed patients to undergo MR imaging exams without potential harm to the device or changes to the device settings. While Mayo Clinic has claimed around 75 percent of patients having an implantable cardiac device may need an MRI in their lifetime, Biotronik has opined around 20 percent of pacemaker patients will need an MRI within the first two years of implant.
The use of cardiac implantable electronic devices in managing patients with heart rhythm conditions has risen in the recent past as these devices have been instrumental in determining any correlation between rhythms and symptoms, helping the diagnosis of a clinically relevant arrhythmia.
Abbott received a go-ahead from the FDA in February 2017 for MR-conditional labeling for its Assurity MRI pacemaker. MRI pacemakers are believed to ward off the time, effort, and inconvenience for a patient with respect to conventional pre- and post-scan pacemaker reprogramming.
The use of magnetic resonance (MR) as an imaging modality in the diagnosis of a slew of conditions has been associated with the soft tissue contrast and the high spatial resolution. There has been a marked rise in the number of patients with implantable pacemaker devices with MRI as some of the factors such as the strength of the magnetic field, gradient power, radiofrequency power, and specific features and components of the implantable devices are being considered by companies. Efforts are being made to make devices more MRI-compatible.
The advent of miniaturized implantable pacemakers has eliminated the requirement of bulky onboard batteries, given they receive energy and commands wirelessly via electromagnetic waves from an external controller. Stakeholders are pushing to the limits to develop pacing devices that can diagnose the pacing needs of the heart in real-time and provide tailored treatments.
It is widely believed pacemakers will be able to reap benefits from artificial intelligence (AI) by learning to identify patterns and make necessary adjustments. The adoption of AI in pacemakers can bring a paradigm shift in the way patients are treated by ensuring seamless patient-specific therapy. Accordingly, smart pacemakers are making inroads to read, recalibrate, self-correct, or adjust and read the heart’s electrical needs to provide a personalized and seamless pacing treatment in “real-time” for each patient.
Pacemakers are increasingly being used for bradyarrhythmias as the cases of sinus bradycardia has increased in recent years. The technological advances of pacemakers, aging population and soaring clinical indications have been contributing to the soaring popularity of these devices in treating bradyarrhythmias.
CRT Pacemakers Grab Eyeballs
While pacemakers are often being used to treat tachycardias, the device has found increased traction as cardiac resynchronization therapy (CRT) pacemakers for patients with heart failure. According to a finding, 40 percent of CRT has been implemented in patients over the age of 80 years. CRT treats heart failure by sending electrical impulses to resynchronize four chambers of the heart.
Given that CRT enhances the efficiency of the heart and boosts blood flow, several clinical studies have revealed a decline in morbidity and hospitalization and have exhibited improvement in the quality of life. The miniaturization of both batteries and electronic components and the advent of wireless technologies have propelled the development of CRT systems.
According to a study, around 80 percent of pacemakers are implanted in the elderly patients, as they are more prone than younger patients to have complications such as bradyarrhythmia. Patients with a clinical history of syncope may require implantation of a pacemaker. As such, upsurge in the elderly population has become an underlying cause fueling the use of pacemakers.
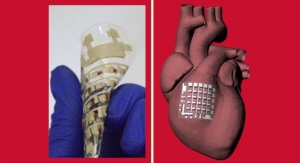
Amidst alleged complication with implantable devices pertaining to leads, leadless pacemakers’ systems have been striving to gain ground. Leads have often been perceived as the weak link in cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) owing to complications from infection. Given leadless pacemakers keep the wire at bay, it is gradually making inroads into the global space.
The U.S. FDA approved the Micra AV on January 21—Medtronic’s new minimally invasive pacer that can apparently tell if the heart’s upper chamber is beating without touching it. Foraying into the leadless pacemaker space, the device comes with a special algorithm and an accelerometer.
In May 2019, FDA issued caution on likely premature battery depletion of certain implantable Medtronic pacemakers. It was reported some of the batteries of these implantable pacemakers drained quickly and without warning patients.
With palpable rise in FDA approval of pacemaker devices, upsurge in elderly population, and uptick in heart diseases, North America has become the major investment hub for manufacturers of pacemaker devices.
With the advent of leadless pacemakers and adoption of AI in medical space, deployment of CRT pacemakers and anti-tachycardia pacemakers has become more apparent. Notably, the development of pacing technology of modern pacemakers and miniaturized technology is increasingly being embraced to help heartbeat into a regular rhythm. Undeniably, the future of the global pacemaker industry looks highly optimistic.
Sunil Jha has been a part of the content industry for close to two years. Having previously worked as a voice over artist and sportswriter, he now focuses on writing articles across numerous topics, ranging from business and technology to trade and finance. With a business-oriented educational background, Sunil brings forth the expertise of intensive research and a strategic approach in his pieces.
Pacemakers have become second to none when it comes to controlling the heartbeat. In common parlance, pacemakers tend to control abnormal heart rhythms and enable the heart to beat at a normal rate using electrical pulses. Notably, pacemakers can rev up a slow heart rhythm, control a fast heart rhythm, and coordinate the chambers of the heart.
Prevalence of arrhythmias has further signified the importance of pacemakers that use low-energy electrical pulses to surmount the faulty electrical signaling. Accordingly, pacemakers can not only control fast or abnormal rhythm, but they also ensure the ventricles contract normally if the atria are quivering and coordinate electrical signaling between the ventricles. Pacemaker devices are also believed to coordinate electrical signaling between the ventricles with the help of cardiac resynchronization therapy (CRT) devices.
Pacemakers Are Here to Stay: MRI Pacemakers Take the World by Storm
The need for pacemakers arises from shortness of breath or that arrhythmias such as tachyarrhythmia and bradyarrhythmia have chances of damaging vital organs. While permanent pacemakers have become the go-to solution, temporary pacemakers are gradually grabbing eyeballs. With an upsurge in the number of people suffering from heart attacks, traction for temporary pacemakers is likely to remain intact in the future.
Reportedly, 1.25 million permanent pacemakers are implanted globally, while around half a million permanent pacemakers were said to be implanted in Europe alone. Temporary pacemakers are used given that the arrhythmias are anticipated to be temporary. Meanwhile, permanent pacemakers find a place for patients with symptomatic bradyarrhythmia or to help kill or prevent tachyarrhythmia.
Technological developments have translated into miniaturization of electronic circuitry and the development of implantable devices. Advances such as device size reduction, internet-based remote monitoring, rate responsiveness, and a notable rise in battery longevity have been linked to implantable pacemakers.
The advent of FDA-cleared MRI pacemakers has taken the world by storm, as these devices have allowed patients to undergo MR imaging exams without potential harm to the device or changes to the device settings. While Mayo Clinic has claimed around 75 percent of patients having an implantable cardiac device may need an MRI in their lifetime, Biotronik has opined around 20 percent of pacemaker patients will need an MRI within the first two years of implant.
The use of cardiac implantable electronic devices in managing patients with heart rhythm conditions has risen in the recent past as these devices have been instrumental in determining any correlation between rhythms and symptoms, helping the diagnosis of a clinically relevant arrhythmia.
Abbott received a go-ahead from the FDA in February 2017 for MR-conditional labeling for its Assurity MRI pacemaker. MRI pacemakers are believed to ward off the time, effort, and inconvenience for a patient with respect to conventional pre- and post-scan pacemaker reprogramming.
The use of magnetic resonance (MR) as an imaging modality in the diagnosis of a slew of conditions has been associated with the soft tissue contrast and the high spatial resolution. There has been a marked rise in the number of patients with implantable pacemaker devices with MRI as some of the factors such as the strength of the magnetic field, gradient power, radiofrequency power, and specific features and components of the implantable devices are being considered by companies. Efforts are being made to make devices more MRI-compatible.
The advent of miniaturized implantable pacemakers has eliminated the requirement of bulky onboard batteries, given they receive energy and commands wirelessly via electromagnetic waves from an external controller. Stakeholders are pushing to the limits to develop pacing devices that can diagnose the pacing needs of the heart in real-time and provide tailored treatments.
It is widely believed pacemakers will be able to reap benefits from artificial intelligence (AI) by learning to identify patterns and make necessary adjustments. The adoption of AI in pacemakers can bring a paradigm shift in the way patients are treated by ensuring seamless patient-specific therapy. Accordingly, smart pacemakers are making inroads to read, recalibrate, self-correct, or adjust and read the heart’s electrical needs to provide a personalized and seamless pacing treatment in “real-time” for each patient.
Pacemakers are increasingly being used for bradyarrhythmias as the cases of sinus bradycardia has increased in recent years. The technological advances of pacemakers, aging population and soaring clinical indications have been contributing to the soaring popularity of these devices in treating bradyarrhythmias.
CRT Pacemakers Grab Eyeballs
While pacemakers are often being used to treat tachycardias, the device has found increased traction as cardiac resynchronization therapy (CRT) pacemakers for patients with heart failure. According to a finding, 40 percent of CRT has been implemented in patients over the age of 80 years. CRT treats heart failure by sending electrical impulses to resynchronize four chambers of the heart.
Given that CRT enhances the efficiency of the heart and boosts blood flow, several clinical studies have revealed a decline in morbidity and hospitalization and have exhibited improvement in the quality of life. The miniaturization of both batteries and electronic components and the advent of wireless technologies have propelled the development of CRT systems.
According to a study, around 80 percent of pacemakers are implanted in the elderly patients, as they are more prone than younger patients to have complications such as bradyarrhythmia. Patients with a clinical history of syncope may require implantation of a pacemaker. As such, upsurge in the elderly population has become an underlying cause fueling the use of pacemakers.
Amidst alleged complication with implantable devices pertaining to leads, leadless pacemakers’ systems have been striving to gain ground. Leads have often been perceived as the weak link in cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) owing to complications from infection. Given leadless pacemakers keep the wire at bay, it is gradually making inroads into the global space.
The U.S. FDA approved the Micra AV on January 21—Medtronic’s new minimally invasive pacer that can apparently tell if the heart’s upper chamber is beating without touching it. Foraying into the leadless pacemaker space, the device comes with a special algorithm and an accelerometer.
In May 2019, FDA issued caution on likely premature battery depletion of certain implantable Medtronic pacemakers. It was reported some of the batteries of these implantable pacemakers drained quickly and without warning patients.
With palpable rise in FDA approval of pacemaker devices, upsurge in elderly population, and uptick in heart diseases, North America has become the major investment hub for manufacturers of pacemaker devices.
With the advent of leadless pacemakers and adoption of AI in medical space, deployment of CRT pacemakers and anti-tachycardia pacemakers has become more apparent. Notably, the development of pacing technology of modern pacemakers and miniaturized technology is increasingly being embraced to help heartbeat into a regular rhythm. Undeniably, the future of the global pacemaker industry looks highly optimistic.
Sunil Jha has been a part of the content industry for close to two years. Having previously worked as a voice over artist and sportswriter, he now focuses on writing articles across numerous topics, ranging from business and technology to trade and finance. With a business-oriented educational background, Sunil brings forth the expertise of intensive research and a strategic approach in his pieces.