MPO Staff11.12.18
The editorial team members of Medical Product Outsourcing and Orthopedic Design & Technology reached out to representatives at several companies exhibiting at Medica/Compamed this year in case you are unable to see them at the event or are not headed to Germany this year. Hopefully, the following questions we served up to Nohora Cardenas, director strategic marketing at Scapa Healthcare, will offer you some additional insight to determine if the company is a potential services partner for you.
MPO: What technology or service are you emphasizing at Medica this year?
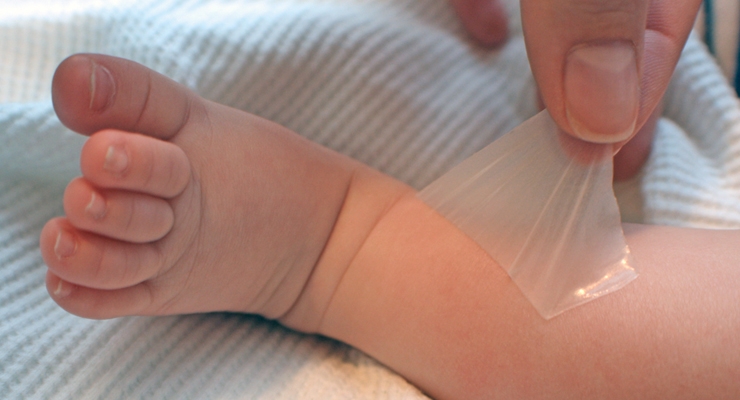
Nohora Cardenas: Scapa Healthcare, a trusted strategic outsource partner of turn-key skin friendly adhesive and topical solutions, will showcase its Scapa Soft-Pro Low Trauma Hydrocolloid (LTH) at Medica. LTH is a proprietary, gentle adhesive with repositionable properties for sensitive skin applications. It has a unique formulation that flows with the skin to prevent discomfort and damage, making it ideal for a full spectrum of users ranging from pediatric and neonatal to geriatric populations. LTH can be gently lifted and reapplied without pulling body hair, while providing secure fixation to the skin. The formulation is biocompatible, latex-free, and gamma stable, allowing for multi-day wear time.
MPO: What’s the most common challenge customers inquire about and how do you address it?
Cardenas: Our customers are faced with pressure to efficiently deliver high quality, innovative medical devices to market quickly. There are two ways they can achieve this: internally, they can heavily invest in differentiating technologies and infrastructure or they can rely on an outsource partner to deliver innovation. Customers struggle with the ability to have in-house capabilities in all areas including manufacturing, regulatory, and technical expertise. With over 20 years of experience in healthcare industry, Scapa Healthcare has established itself as a trusted strategic outsource partner for leading global healthcare companies, delivering a full suite of global capabilities.
MPO: If you could give one piece of advice to companies seeking a manufacturing partner before they make a decision, what would it be?
Cardenas: With the ever-changing complexities of the healthcare system, it is difficult for companies to have in-house expertise in every single field. Outsource companies can offer a high level of expertise to expedite the development process and deliver innovative products to market rapidly. A strategic outsource partner should be seen not only as an extension of their own manufacturing operation, but also an integral part of their R&D team to enhance their development capabilities.
MPO: What are the forces driving medical device manufacturers to seek your technology/services over doing it in-house?
Cardenas: Large healthcare companies, particularly publicly traded organizations, face significant pressure to consistently deliver positive results. One way to achieve this is to keep investment costs low and free up resources. Healthcare companies want to focus their resources on their strengths – distribution, marketing and commercialization. Outsource partners can more efficiently manage innovation, design, development and manufacturing. By focusing on each party’s strengths, outsource companies become an additional arm for leading healthcare brands, resulting in a mutually beneficial relationship.
Scapa Healthcare has two Turn-Key Centers of Excellence in the United States and Europe that house turn-key capabilities to take a product from concept to commercialization. All global facilities are ISO-certified and FDA registered with environmentally controlled rooms for full-scale production of finished goods. Our partners can rely on our turn-key solutions and experts to design, develop and deliver the innovative products that can differentiate them in the marketplace.
MPO: In what ways is your company able to aid in getting a product (project) to market faster?
Cardenas: Scapa Healthcare has invested heavily to expand its capabilities to be able to offer global turn-key solutions to its partners. In 2018 alone, we have acquired a topical skin care solutions company in Dallas, Texas, and a turn-key manufacturing facility in Gargrave, England. Our highly qualified teams manage the entire design, develop and manufacturing process, including regulatory and logistic services. Combining our streamlined processes with dedicated project teams, Scapa Healthcare can help deliver innovative products to market faster, ultimately enhancing our partners’ competitive position in the market.
Scapa Healthcare is located at Medica/Compamed in Hall 6, Booth/Stand H48.
MPO: What technology or service are you emphasizing at Medica this year?
Nohora Cardenas: Scapa Healthcare, a trusted strategic outsource partner of turn-key skin friendly adhesive and topical solutions, will showcase its Scapa Soft-Pro Low Trauma Hydrocolloid (LTH) at Medica. LTH is a proprietary, gentle adhesive with repositionable properties for sensitive skin applications. It has a unique formulation that flows with the skin to prevent discomfort and damage, making it ideal for a full spectrum of users ranging from pediatric and neonatal to geriatric populations. LTH can be gently lifted and reapplied without pulling body hair, while providing secure fixation to the skin. The formulation is biocompatible, latex-free, and gamma stable, allowing for multi-day wear time.
MPO: What’s the most common challenge customers inquire about and how do you address it?
Cardenas: Our customers are faced with pressure to efficiently deliver high quality, innovative medical devices to market quickly. There are two ways they can achieve this: internally, they can heavily invest in differentiating technologies and infrastructure or they can rely on an outsource partner to deliver innovation. Customers struggle with the ability to have in-house capabilities in all areas including manufacturing, regulatory, and technical expertise. With over 20 years of experience in healthcare industry, Scapa Healthcare has established itself as a trusted strategic outsource partner for leading global healthcare companies, delivering a full suite of global capabilities.
MPO: If you could give one piece of advice to companies seeking a manufacturing partner before they make a decision, what would it be?
Cardenas: With the ever-changing complexities of the healthcare system, it is difficult for companies to have in-house expertise in every single field. Outsource companies can offer a high level of expertise to expedite the development process and deliver innovative products to market rapidly. A strategic outsource partner should be seen not only as an extension of their own manufacturing operation, but also an integral part of their R&D team to enhance their development capabilities.
MPO: What are the forces driving medical device manufacturers to seek your technology/services over doing it in-house?
Cardenas: Large healthcare companies, particularly publicly traded organizations, face significant pressure to consistently deliver positive results. One way to achieve this is to keep investment costs low and free up resources. Healthcare companies want to focus their resources on their strengths – distribution, marketing and commercialization. Outsource partners can more efficiently manage innovation, design, development and manufacturing. By focusing on each party’s strengths, outsource companies become an additional arm for leading healthcare brands, resulting in a mutually beneficial relationship.
Scapa Healthcare has two Turn-Key Centers of Excellence in the United States and Europe that house turn-key capabilities to take a product from concept to commercialization. All global facilities are ISO-certified and FDA registered with environmentally controlled rooms for full-scale production of finished goods. Our partners can rely on our turn-key solutions and experts to design, develop and deliver the innovative products that can differentiate them in the marketplace.
MPO: In what ways is your company able to aid in getting a product (project) to market faster?
Cardenas: Scapa Healthcare has invested heavily to expand its capabilities to be able to offer global turn-key solutions to its partners. In 2018 alone, we have acquired a topical skin care solutions company in Dallas, Texas, and a turn-key manufacturing facility in Gargrave, England. Our highly qualified teams manage the entire design, develop and manufacturing process, including regulatory and logistic services. Combining our streamlined processes with dedicated project teams, Scapa Healthcare can help deliver innovative products to market faster, ultimately enhancing our partners’ competitive position in the market.
Scapa Healthcare is located at Medica/Compamed in Hall 6, Booth/Stand H48.