Theranica09.08.20
Theranica, a prescribed digital therapeutics company developing advanced electroceuticals for migraine and other pain conditions, today announced that it has been granted a CE mark for its Nerivio device for acute treatment of migraine, a little more than a year after receiving FDA clearance in the United States.
This milestone allows the award-winning wearable therapeutic device to be marketed in select countries in Europe, as part of the company’s roll-out plan, starting in 2021. The CE mark was granted under the new MDR European regulation.
“This is great news for millions of migraine patients in Europe, many of whom are seeking a non-pharmacological therapy for their debilitating migraines,” said Messoud Ashina, MD, professor of neurology and chief physician at the Department of Neurology, Rigshospitalet in Copenhagen, Denmark and current president of the International Headache Society. “It is important to expand the toolbox available for healthcare providers and patients in their effort to find the best therapy for each individual.”
An estimated 41 million Europeans suffer from migraine, costing the European economy more than 50 billion Euros a year. Far more than just a headache, migraine can also be accompanied by symptoms such as nausea, numbness, sensitivity to light and sound and confusion.
“Nerivio combines clinical efficacy comparable to prescription drugs, with a high safety profile,” said Theranica CEO Alon Ironi. “After its successful launch in the US, Nerivio will soon provide European migraine patients with the option of a non-pharmacological treatment that can keep them safe from medication overuse headache. The ease of use, focused on rapid return to normal functioning, combined with the digital health emphasis as a smartphone-controlled wearable, is a fresh addition to the arsenal of migraine therapies.”
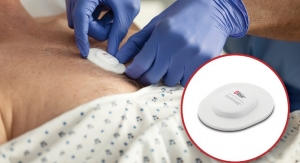
Nerivio is the first smartphone-controlled wearable device for the acute treatment of migraine. Placed on the upper arm at the onset of a migraine attack, the prescribed device uses remote electrical neuromodulation to wirelessly stimulate a conditioned pain modulation response to mitigate pain. A corresponding Nerivio app also keeps track of migraine episodes and provides analytics that a patient can share with their doctor, to help monitor and tailor treatment.
Those interested in receiving more information about obtaining Nerivio can submit questions here.
This milestone allows the award-winning wearable therapeutic device to be marketed in select countries in Europe, as part of the company’s roll-out plan, starting in 2021. The CE mark was granted under the new MDR European regulation.
“This is great news for millions of migraine patients in Europe, many of whom are seeking a non-pharmacological therapy for their debilitating migraines,” said Messoud Ashina, MD, professor of neurology and chief physician at the Department of Neurology, Rigshospitalet in Copenhagen, Denmark and current president of the International Headache Society. “It is important to expand the toolbox available for healthcare providers and patients in their effort to find the best therapy for each individual.”
An estimated 41 million Europeans suffer from migraine, costing the European economy more than 50 billion Euros a year. Far more than just a headache, migraine can also be accompanied by symptoms such as nausea, numbness, sensitivity to light and sound and confusion.
“Nerivio combines clinical efficacy comparable to prescription drugs, with a high safety profile,” said Theranica CEO Alon Ironi. “After its successful launch in the US, Nerivio will soon provide European migraine patients with the option of a non-pharmacological treatment that can keep them safe from medication overuse headache. The ease of use, focused on rapid return to normal functioning, combined with the digital health emphasis as a smartphone-controlled wearable, is a fresh addition to the arsenal of migraine therapies.”
Nerivio is the first smartphone-controlled wearable device for the acute treatment of migraine. Placed on the upper arm at the onset of a migraine attack, the prescribed device uses remote electrical neuromodulation to wirelessly stimulate a conditioned pain modulation response to mitigate pain. A corresponding Nerivio app also keeps track of migraine episodes and provides analytics that a patient can share with their doctor, to help monitor and tailor treatment.
Those interested in receiving more information about obtaining Nerivio can submit questions here.