Business Wire08.12.20
Baxter International Inc. received Emergency Use Authorizations (EUAs) from the U.S. Food and Drug Administration (FDA) for the company’s HF20 Set and ST Set used in continuous renal replacement therapy (CRRT). Under its EUA, the HF20 Set is authorized to deliver CRRT to treat patients of low weight (8-20 kg) and low blood volume who cannot tolerate a larger extracorporeal circuit volume in an acute care environment during the COVID-19 pandemic. The ST Set is authorized for use under its EUA to provide CRRT to treat patients in an acute care environment during the COVID-19 pandemic. Both the HF20 Set and ST Set can be used with the Prismaflex or PrisMax control units (monitors).
“With the continued need for CRRT products, the addition of the HF20 Set and ST Set offers healthcare providers and hospitals greater flexibility to meet the varying needs of patients, while making more CRRT sets available in the U.S.,” said Reaz Rasul, general manager of Baxter’s Acute Therapies business. “With the HF20 Set, we are thrilled to help expand access to CRRT to low weight patients during the COVID-19 pandemic.”
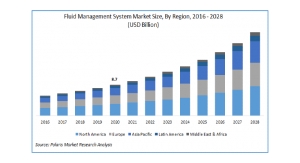
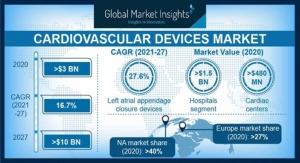
Acute kidney injury (AKI), a potentially life-threatening condition where the kidneys suddenly stop working and fluid and uremic toxins build up in the body, is one of many complications affecting COVID-19 patients. A recent meta-analysis of 20 studies evaluated more than 13,000 hospitalized COVID-19 patients, 43 percent of whom were in the intensive care unit or had severe infection, and found that median AKI prevalence was 17 percent, with a range of 0.5 percent - 80.3 percent.1 CRRT mimics many of the functions of the natural kidney and is the cornerstone of treatment in patients with severe AKI.2 During CRRT, the patient’s blood passes through a special filter where fluid and uremic toxins are removed before the cleaned blood is returned to the body.
The HF20 Set offers low extracorporeal blood volumes (58mL) and the PolyArylEtherSulfone (PAES) filter membrane to clear uremic toxins and manage fluid overload. The ST Set includes three sizes that allow the healthcare provider to choose the most appropriate option for the patient. It features Baxter’s proprietary AN69 membrane, which can adsorb toxins with basic residues on the surface by means of ionic interactions.
Both the HF20 Set and ST Set are pre-connected disposable sets used with Baxter’s Prismaflex and PrisMax monitors, and work with all CRRT modalities and most commonly used anti-coagulants. The HF20 Set and ST Set have not been cleared or approved by FDA in the U.S. but have been in use for more than 10 years across countries in Europe. A limited initial shipment will be available in the U.S. as soon as possible, with more significant production ramping up throughout the coming weeks and months.
Reference
1 Shelief Y. Robbins-Juarez, BA, Long Qian, MD, Kristen L. King, MPH, Jacob S. Stevens, MD, S. Ali Husain, MD, MPH, Jai Radhakrishnan, MD, Sumit Mohan, MD, MPH. A Systematic Review and Meta-Analysis Of Outcomes for Patients with COVID-19 and Acute Kidney Injury. Kidney Int Rep. Published online June 24, 2020
“With the continued need for CRRT products, the addition of the HF20 Set and ST Set offers healthcare providers and hospitals greater flexibility to meet the varying needs of patients, while making more CRRT sets available in the U.S.,” said Reaz Rasul, general manager of Baxter’s Acute Therapies business. “With the HF20 Set, we are thrilled to help expand access to CRRT to low weight patients during the COVID-19 pandemic.”
Acute kidney injury (AKI), a potentially life-threatening condition where the kidneys suddenly stop working and fluid and uremic toxins build up in the body, is one of many complications affecting COVID-19 patients. A recent meta-analysis of 20 studies evaluated more than 13,000 hospitalized COVID-19 patients, 43 percent of whom were in the intensive care unit or had severe infection, and found that median AKI prevalence was 17 percent, with a range of 0.5 percent - 80.3 percent.1 CRRT mimics many of the functions of the natural kidney and is the cornerstone of treatment in patients with severe AKI.2 During CRRT, the patient’s blood passes through a special filter where fluid and uremic toxins are removed before the cleaned blood is returned to the body.
The HF20 Set offers low extracorporeal blood volumes (58mL) and the PolyArylEtherSulfone (PAES) filter membrane to clear uremic toxins and manage fluid overload. The ST Set includes three sizes that allow the healthcare provider to choose the most appropriate option for the patient. It features Baxter’s proprietary AN69 membrane, which can adsorb toxins with basic residues on the surface by means of ionic interactions.
Both the HF20 Set and ST Set are pre-connected disposable sets used with Baxter’s Prismaflex and PrisMax monitors, and work with all CRRT modalities and most commonly used anti-coagulants. The HF20 Set and ST Set have not been cleared or approved by FDA in the U.S. but have been in use for more than 10 years across countries in Europe. A limited initial shipment will be available in the U.S. as soon as possible, with more significant production ramping up throughout the coming weeks and months.
Reference
1 Shelief Y. Robbins-Juarez, BA, Long Qian, MD, Kristen L. King, MPH, Jacob S. Stevens, MD, S. Ali Husain, MD, MPH, Jai Radhakrishnan, MD, Sumit Mohan, MD, MPH. A Systematic Review and Meta-Analysis Of Outcomes for Patients with COVID-19 and Acute Kidney Injury. Kidney Int Rep. Published online June 24, 2020