PR Newswire07.24.20
MiraVista Diagnostics LLC, the leader in diagnostic solutions for serious fungal infections and endemic mycosis, announced the release of its new CE Marked immunochromatographic lateral flow assay for the qualitative detection of Histoplasma antigen in urine samples.
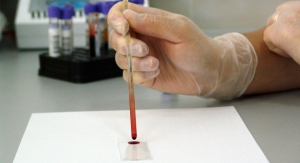
The MiraVista Histoplasma Urine Antigen Lateral Flow Assay (LFA) is the first rapid diagnostic test that is highly sensitive and specific for the detection of Histoplasma antigen in urine and can be utilized in low complexity clinical settings without additional equipment.
The MiraVista Histoplasma Urine Antigen LFA provides results in 40 minutes and a kit includes all necessary reagents, devices, pipettes and controls to produce 25 patient results. The LFA is manufactured and controlled under a quality management system certified to ISO 13485.
"Histoplasmosis is a leading cause of morbidity and death in persons living with HIV/AIDS in many resource limited countries," said Dr. L. Joseph Wheat, founder and medical director of MiraVista Diagnostics. "The outcome in the United States, where antigen detection is the most common method for diagnosis of disseminated histoplasmosis in immunocompromised patients, is excellent. The poor outcome in resourced limited countries is largely caused by delay in diagnosis. Current antigen detection methods are not widely available in these countries, are insensitive and do not provide timely results. The new lateral flow assay offers a rapid and cost-efficient method for diagnosis and the possibility of reducing morbidity and death caused by histoplasmosis."
"Achieving CE marking for the Histoplasma antigen lateral flow device continues the Company's breakthrough diagnostic tests for detection of dimorphic fungi and solidifies our position as a leader in accurate diagnosis of these deadly infections," commented Dr. Slava Elagin, chief operations officer at MiraVista Diagnostics. "This is the first commercial launch of an IVD product in our history, which creates new business opportunities as we continue to diversify from a clinical laboratory organization into manufacturer of IVD products. MiraVista will be working with global partners to provide innovative products that truly address unmet needs for diagnosis of fungal infections."
The MiraVista Histoplasma Urine Antigen Lateral Flow Assay (LFA) is the first rapid diagnostic test that is highly sensitive and specific for the detection of Histoplasma antigen in urine and can be utilized in low complexity clinical settings without additional equipment.
The MiraVista Histoplasma Urine Antigen LFA provides results in 40 minutes and a kit includes all necessary reagents, devices, pipettes and controls to produce 25 patient results. The LFA is manufactured and controlled under a quality management system certified to ISO 13485.
"Histoplasmosis is a leading cause of morbidity and death in persons living with HIV/AIDS in many resource limited countries," said Dr. L. Joseph Wheat, founder and medical director of MiraVista Diagnostics. "The outcome in the United States, where antigen detection is the most common method for diagnosis of disseminated histoplasmosis in immunocompromised patients, is excellent. The poor outcome in resourced limited countries is largely caused by delay in diagnosis. Current antigen detection methods are not widely available in these countries, are insensitive and do not provide timely results. The new lateral flow assay offers a rapid and cost-efficient method for diagnosis and the possibility of reducing morbidity and death caused by histoplasmosis."
"Achieving CE marking for the Histoplasma antigen lateral flow device continues the Company's breakthrough diagnostic tests for detection of dimorphic fungi and solidifies our position as a leader in accurate diagnosis of these deadly infections," commented Dr. Slava Elagin, chief operations officer at MiraVista Diagnostics. "This is the first commercial launch of an IVD product in our history, which creates new business opportunities as we continue to diversify from a clinical laboratory organization into manufacturer of IVD products. MiraVista will be working with global partners to provide innovative products that truly address unmet needs for diagnosis of fungal infections."