Business Wire07.24.20
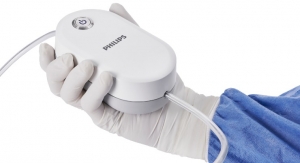
Palliare has received 510(k) clearance from the FDA for its patented EVA15 insufflator and smoke evacuation system. EVA15 is a novel insufflator designed to meet the demanding insufflation requirements of endoscopic and robotic surgery, while offering integrated surgical smoke evacuation capability, filtering the hazardous smoke particles produced during laparoscopic surgery and removing them from the operating room. The built-in smoke evacuation capability also allows EVA15 to reduce device footprint in the OR by 50 percent while significantly reducing the cost of each procedure.
“EVA15 is the optimal solution for insufflation and surgical smoke evacuation, with one device managing two of the most critical needs for these types of surgical procedures,” said Caroline O’Dea, Managing Director, Palliare. “We are excited to receive FDA 510(k) clearance for EVA15, marking a crucial step toward bringing this important product to market later this year.”
The dangers associated with surgical smoke inhalation have been well documented, leading recently to a number of US states passing legislation mandating the use of such smoke evacuation systems to protect the safety of operating room staff. The American Association of periOperative Registered Nurses (AORN) estimates that being in an operating room for just one day has the potential to expose operating staff to surgical smoke that is equivalent to smoking 27 to 30 unfiltered cigarettes. The EVA15 laparoscopic insufflator provides pedal-activated smoke evacuation, and has the unique capability of connecting directly to the OR scavenging system, ensuring that smoke is completely removed from the operating room and not re-circulated.
Palliare is also pleased to announce two appointments to its Board of Directors. With more than 30 years of experience in the surgical industry, Scott D. Flora will serve as Chairman of the Board. Flora recently served as CEO of Invuity prior to its acquisition by Stryker, and has previously served as President, Global Business Unit, Surgical Devices at Covidien (NYSE:MDT). Additionally, leading Irish Medtech entrepreneur John O’Shaughnessy will serve on Palliare’s Board of Directors. John was a co-founder and Chairman of Neuravi prior to its acquisition by Johnson & Johnson, and co-founder and CEO of MedNova prior to its acquisition by Abbott.
“We are delighted to have Scott Flora and John O’Shaughnessy join the Palliare Board,” said O’Dea. “Palliare will greatly benefit from their significant experience as we prepare for the EVA15 product launch.”
“EVA15 is the optimal solution for insufflation and surgical smoke evacuation, with one device managing two of the most critical needs for these types of surgical procedures,” said Caroline O’Dea, Managing Director, Palliare. “We are excited to receive FDA 510(k) clearance for EVA15, marking a crucial step toward bringing this important product to market later this year.”
The dangers associated with surgical smoke inhalation have been well documented, leading recently to a number of US states passing legislation mandating the use of such smoke evacuation systems to protect the safety of operating room staff. The American Association of periOperative Registered Nurses (AORN) estimates that being in an operating room for just one day has the potential to expose operating staff to surgical smoke that is equivalent to smoking 27 to 30 unfiltered cigarettes. The EVA15 laparoscopic insufflator provides pedal-activated smoke evacuation, and has the unique capability of connecting directly to the OR scavenging system, ensuring that smoke is completely removed from the operating room and not re-circulated.
Palliare is also pleased to announce two appointments to its Board of Directors. With more than 30 years of experience in the surgical industry, Scott D. Flora will serve as Chairman of the Board. Flora recently served as CEO of Invuity prior to its acquisition by Stryker, and has previously served as President, Global Business Unit, Surgical Devices at Covidien (NYSE:MDT). Additionally, leading Irish Medtech entrepreneur John O’Shaughnessy will serve on Palliare’s Board of Directors. John was a co-founder and Chairman of Neuravi prior to its acquisition by Johnson & Johnson, and co-founder and CEO of MedNova prior to its acquisition by Abbott.
“We are delighted to have Scott Flora and John O’Shaughnessy join the Palliare Board,” said O’Dea. “Palliare will greatly benefit from their significant experience as we prepare for the EVA15 product launch.”