PR Newswire07.24.20
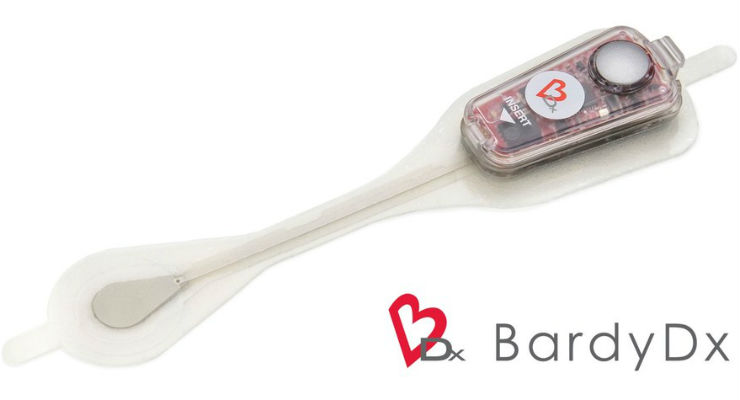
Bardy Diagnostics Inc., a provider of ambulatory cardiac monitoring technologies and custom data solutions, has received CE mark certification for the 14-Day version of the Carnation Ambulatory Monitor (CAM) patch, the industry's only P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device. The 14-Day CAM patch provides clinicians greater flexibility to monitor their patients over a longer period and expands the portfolio of existing 2-Day and 7-Day CAM patches currently offered by BardyDx. The 14-Day CAM patch received 510(k) clearance from the U.S. Food and Drug Administration in September 2019 and Health Canada clearance in February 2020.
"CE marking of the 14-Day CAM patch is a significant corporate milestone and a testament to the quality of the CAM patch and BardyDx's compliance with all applicable European health, safety, performance and environmental requirements," said Ed Vertatschitsch, chief operating officer. Ken Nelson, chief commercial officer added, "Along with our Health Canada Medical Device License, the ability to distribute a CE-marked 14-CAM patch outside of the United States is one more step towards our P-wave centric detection and analysis technology becoming the global standard of care in long-term cardiac monitoring." The CAM patch is distributed by Dot Medical in the United Kingdom and by JNC Medical in Canada.
The 14-Day CAM patch provides up to double the duration of the current 7-Day CAM patch, providing clinicians a greater opportunity to detect less-frequently occurring arrhythmias and to better inform decisions and prioritize care. The clinical value of the BardyDx P-wave centric detection technology was reported in a study published in the American Heart Journal describing the results of a head-to-head comparison with the iRhythm Zio XT patch. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," (Am Heart J 2018; 203:109-117) concluded that the BardyDx CAM Patch identified 40 percent more arrhythmias and resulted in better, more informed clinical decision-making in 41 percent of patients as compared to the iRhythm Zio XT patch.
BardyDx also announced the expansion of its Home Enrollment Program. Typically, the CAM patch is applied to a patient in a clinic setting, but the rapidly evolving COVID-19 public health emergency has increased the use of telehealth and direct to patient delivery of remote monitoring devices, particularly in the cardiac monitoring space, to reduce resource burdens on healthcare facilities and risk of exposure of patients and their caregivers to emerging or other infectious diseases.
"We are all fighting the COVID-19 pandemic together. To help address the evolving needs of physicians and patients in this public health emergency, facilitate the rapid transition to telehealth for clinics around the country and to ensure uninterrupted access to our CAM patch, our team has worked around the clock to increase our manufacturing capacity and expand our internal business operations to enable patients to receive and apply our disposable, single-use CAM patch at home," said Nelson. "The home application program eliminates the need for in-person patient and healthcare provider contact, reduces the potential for exposure to COVID-19, streamlines care, and allows healthcare personnel who are already resource-constrained to focus on other areas of critical need."
The CAM patch is currently used in hospital protocols to help physicians better identify and understand arrhythmias that may be related to COVID-19, including in a number of clinical trials of COVID-19 patients at academic centers across the United States who are being treated with Azithromycin in order to monitor for QT interval prolongation, which may lead to potentially life threatening cardiac arrhythmias.
"CE marking of the 14-Day CAM patch is a significant corporate milestone and a testament to the quality of the CAM patch and BardyDx's compliance with all applicable European health, safety, performance and environmental requirements," said Ed Vertatschitsch, chief operating officer. Ken Nelson, chief commercial officer added, "Along with our Health Canada Medical Device License, the ability to distribute a CE-marked 14-CAM patch outside of the United States is one more step towards our P-wave centric detection and analysis technology becoming the global standard of care in long-term cardiac monitoring." The CAM patch is distributed by Dot Medical in the United Kingdom and by JNC Medical in Canada.
The 14-Day CAM patch provides up to double the duration of the current 7-Day CAM patch, providing clinicians a greater opportunity to detect less-frequently occurring arrhythmias and to better inform decisions and prioritize care. The clinical value of the BardyDx P-wave centric detection technology was reported in a study published in the American Heart Journal describing the results of a head-to-head comparison with the iRhythm Zio XT patch. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," (Am Heart J 2018; 203:109-117) concluded that the BardyDx CAM Patch identified 40 percent more arrhythmias and resulted in better, more informed clinical decision-making in 41 percent of patients as compared to the iRhythm Zio XT patch.
BardyDx also announced the expansion of its Home Enrollment Program. Typically, the CAM patch is applied to a patient in a clinic setting, but the rapidly evolving COVID-19 public health emergency has increased the use of telehealth and direct to patient delivery of remote monitoring devices, particularly in the cardiac monitoring space, to reduce resource burdens on healthcare facilities and risk of exposure of patients and their caregivers to emerging or other infectious diseases.
"We are all fighting the COVID-19 pandemic together. To help address the evolving needs of physicians and patients in this public health emergency, facilitate the rapid transition to telehealth for clinics around the country and to ensure uninterrupted access to our CAM patch, our team has worked around the clock to increase our manufacturing capacity and expand our internal business operations to enable patients to receive and apply our disposable, single-use CAM patch at home," said Nelson. "The home application program eliminates the need for in-person patient and healthcare provider contact, reduces the potential for exposure to COVID-19, streamlines care, and allows healthcare personnel who are already resource-constrained to focus on other areas of critical need."
The CAM patch is currently used in hospital protocols to help physicians better identify and understand arrhythmias that may be related to COVID-19, including in a number of clinical trials of COVID-19 patients at academic centers across the United States who are being treated with Azithromycin in order to monitor for QT interval prolongation, which may lead to potentially life threatening cardiac arrhythmias.