Business Wire07.09.20
Pulmonx Corporation, a commercial-stage medical technology company that provides a minimally-invasive treatment for patients with severe emphysema, has announced a $66 million financing led by Ally Bridge Group, a global life science investor. The financing also attracted new investors Adage Capital Management, HealthQuest Capital, Partner Fund Management, and Rock Springs Capital, as well as existing investors. In addition, $17 million in growth capital was secured through CIBC Innovation Banking to refinance an existing debt facility.
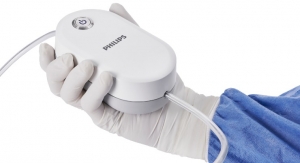
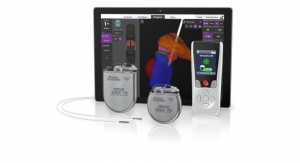
Proceeds from this financing will ensure that Pulmonx is well-capitalized to support the continued global commercial expansion of the Zephyr Valve System, the first U.S. Food and Drug Administration-approved minimally-invasive treatment option for severe emphysema, a form of COPD. The Zephyr Valve procedure, done through a simple bronchoscopy with no incision or tissue resection, is clinically proven to improve patients’ breathing, exercise capacity, and quality of life, without the risks of major surgery.1
“Ally Bridge is pleased to support Pulmonx in this oversubscribed financing,” said Frank Yu, Founder, CEO and CIO of Ally Bridge Group. “The combination of Pulmonx’ Zephyr Valve System, patient assessment tools, established reimbursement programs, and global commercial footprint positions it as the world leader in interventional COPD procedures.”
Emphysema is a progressive and life-threatening form of Chronic Obstructive Pulmonary Disease (COPD) and represents about 25 percent of all COPD patients. In the United States, COPD is the third leading cause of death and is expected to be associated with approximately $49 billion in direct medical costs in 2020. Emphysema is a debilitating and life-threatening disease that progressively destroys lung tissue, resulting in a diminishing ability to breathe and engage in the most basic daily activities, leading to further deconditioning and to a high mortality rate. It is estimated that more than 1.2 million severe emphysema patients in the United States, Europe, and Japan are candidates for the Zephyr Valve treatment. Medical therapy delivers limited benefit in later stage patients, and because of the high risks and limited availability, surgical options are only available for a narrow patient population.
“We are pleased to be able to accelerate our efforts to bring our landmark technology to severe emphysema patients who need it,” said Glen French, CEO of Pulmonx. “The recently secured financing will ensure we are able to continue to scale our commercialization efforts to meet the global demand for our Zephyr Valve System, as well as continue R&D efforts on new minimally-invasive pulmonary treatments.”
Bronchoscopic lung volume reduction with the Zephyr Valve is a one-time procedure performed through a bronchoscope, which requires no cutting or incisions. During the procedure, an average of four valves are placed in the airways to block off a diseased portion of the lung. The target area then reduces in size and allows adjacent healthier lung tissue to expand and function more efficiently. This results in patients being able to breathe more easily and experience less shortness of breath. Many patients treated with the Zephyr Valves have reported immediate relief and the ability to go back to doing everyday tasks with greater ease within weeks of treatment. Data from four published randomized controlled clinical studies have proven that the Zephyr Valve delivers significant and persistent improvement in pulmonary function, exercise capacity, breathlessness, and quality of life with less morbidity and mortality than surgical treatment options.2,3,4,5 These data have led to the inclusion of Zephyr Valves in several national and global treatment guidelines including the U.K.’s National Institute for Health and Care Excellent (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) which gives endobronchial valves a level ‘A’ evidence rating, the highest rating possible.
References
1 Am J Respir Crit Care Med Vol 198, Issue 9, pp 1151–1164, Nov 1, 2018.
2 Am J Respir Crit Care Med Vol 198, Issue 9, pp 1151–1164 (2018).
3 Am J Respir Crit Care Med, Vol 196, Issue 12, pp 1535-1543 (2015).
4 Am J Respir Crit Care Med, Vol 194 Issue 9, pp 1073-1082 (2016).
5 N Engl J Med, Vol 373, Issue 24, pp 2325-2335 (2015).
Proceeds from this financing will ensure that Pulmonx is well-capitalized to support the continued global commercial expansion of the Zephyr Valve System, the first U.S. Food and Drug Administration-approved minimally-invasive treatment option for severe emphysema, a form of COPD. The Zephyr Valve procedure, done through a simple bronchoscopy with no incision or tissue resection, is clinically proven to improve patients’ breathing, exercise capacity, and quality of life, without the risks of major surgery.1
“Ally Bridge is pleased to support Pulmonx in this oversubscribed financing,” said Frank Yu, Founder, CEO and CIO of Ally Bridge Group. “The combination of Pulmonx’ Zephyr Valve System, patient assessment tools, established reimbursement programs, and global commercial footprint positions it as the world leader in interventional COPD procedures.”
Emphysema is a progressive and life-threatening form of Chronic Obstructive Pulmonary Disease (COPD) and represents about 25 percent of all COPD patients. In the United States, COPD is the third leading cause of death and is expected to be associated with approximately $49 billion in direct medical costs in 2020. Emphysema is a debilitating and life-threatening disease that progressively destroys lung tissue, resulting in a diminishing ability to breathe and engage in the most basic daily activities, leading to further deconditioning and to a high mortality rate. It is estimated that more than 1.2 million severe emphysema patients in the United States, Europe, and Japan are candidates for the Zephyr Valve treatment. Medical therapy delivers limited benefit in later stage patients, and because of the high risks and limited availability, surgical options are only available for a narrow patient population.
“We are pleased to be able to accelerate our efforts to bring our landmark technology to severe emphysema patients who need it,” said Glen French, CEO of Pulmonx. “The recently secured financing will ensure we are able to continue to scale our commercialization efforts to meet the global demand for our Zephyr Valve System, as well as continue R&D efforts on new minimally-invasive pulmonary treatments.”
Bronchoscopic lung volume reduction with the Zephyr Valve is a one-time procedure performed through a bronchoscope, which requires no cutting or incisions. During the procedure, an average of four valves are placed in the airways to block off a diseased portion of the lung. The target area then reduces in size and allows adjacent healthier lung tissue to expand and function more efficiently. This results in patients being able to breathe more easily and experience less shortness of breath. Many patients treated with the Zephyr Valves have reported immediate relief and the ability to go back to doing everyday tasks with greater ease within weeks of treatment. Data from four published randomized controlled clinical studies have proven that the Zephyr Valve delivers significant and persistent improvement in pulmonary function, exercise capacity, breathlessness, and quality of life with less morbidity and mortality than surgical treatment options.2,3,4,5 These data have led to the inclusion of Zephyr Valves in several national and global treatment guidelines including the U.K.’s National Institute for Health and Care Excellent (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) which gives endobronchial valves a level ‘A’ evidence rating, the highest rating possible.
References
1 Am J Respir Crit Care Med Vol 198, Issue 9, pp 1151–1164, Nov 1, 2018.
2 Am J Respir Crit Care Med Vol 198, Issue 9, pp 1151–1164 (2018).
3 Am J Respir Crit Care Med, Vol 196, Issue 12, pp 1535-1543 (2015).
4 Am J Respir Crit Care Med, Vol 194 Issue 9, pp 1073-1082 (2016).
5 N Engl J Med, Vol 373, Issue 24, pp 2325-2335 (2015).