PRNewswire06.15.20
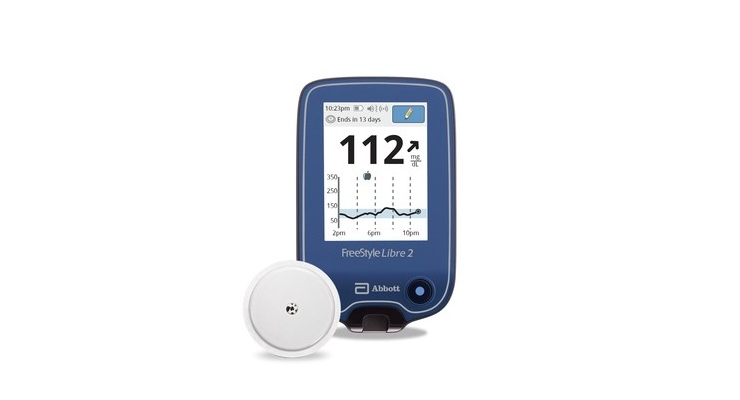
The U.S. Food and Drug Administration (FDA) has cleared Abbott’s next-generation FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system for adults and children ages 4 and older with diabetes.
According to Abbott, this is the only iCGM system with optional real-time alarms that measures glucose levels every minute, meeting the highest level of accuracy standards5 over 14 days, including superior day one1 accuracy compared to the other iCGM and excellent accuracy and alarm performance at low end glucose levels.6 With a 14-day wear time, the FreeStyle Libre 2 system “is the longest-lasting, self-applied iCGM sensor currently available, eliminating the need for fingersticks2– and priced at a third of the cost of other CGM systems.”4
"We're thrilled to bring our next generation technology on our world-leading sensing platform to both children and adults with diabetes in the U.S.," said Jared Watkin, senior vice president, diabetes care, Abbott. "With unsurpassed 14-day accuracy and enhanced features including optional alarms at a fraction of the cost of other CGMs, Abbott's FreeStyle Libre 2 system will change the future of diabetes care in the U.S. the same way it has around the globe."
Using Bluetooth technology, the FreeStyle Libre 2 system automatically alerts users when their glucose is high or low without needing to scan the sensor. Users also have the option of turning off the customizable, real-time alarms.7 The system has a combined mean absolute relative difference (MARD), a measurement of performance for CGMs, of 9.3% (9.2% for adults and 9.7% for pediatrics).
"Innovations like FreeStyle Libre 2 will change the way people manage their diabetes, especially among children," said Larry Kurt Midyett, M.D., pediatric endocrinologist, Midwest Women's and Children's Specialty Group. "Using this technology can improve time in optimal glucose range and lower HbA1c because we can get a full picture of what a child's glucose levels are doing without having to disrupt their play or sleep with painful fingersticks. The alarms are a bonus because they provide parents a level of reassurance."
The FreeStyle Libre 2 next-generation sensor is worn on the back of the upper arm for up to 14 days and measures glucose every minute to help users and their healthcare providers make informed treatment decisions. With a one-second scan using a handheld reader, users can see their glucose reading, trend arrow and eight-hour history. It is also designed for use with a mobile app, which Abbott is working to bring to the U.S. market.
The FreeStyle Libre 2 system will be available in the coming weeks at participating pharmacies and durable medical equipment suppliers (DMEs) across the U.S. Abbott will offer the new system at the same price as the currently available FreeStyle Libre 14 day system, which was approved by the FDA in July 2018.
References:
1 Data on file, Abbott Diabetes Care.
2 Fingersticks are required if your glucose alarms and readings do not match symptoms or when you see Check Blood Glucose symbol during the first 12 hours.
3 Eeg-Olofsson, Katarina et al. Real-world study of FreeStyle Libre system among adults with Type 1 and Type 2 diabetes within the Swedish National Diabetes Register. Poster presented at ATTD, February 2020.
4 Based on a comparison of list prices of the FreeStyle Libre 14 day system versus competitors' CGM systems. FreeStyle Libre 2 system will be list priced the same rate as FreeStyle Libre 14 day system. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
5 Based on FDA iCGM special controls.
6 FreeStyle Libre 2 User Manual. Based on low glucose alarms set at 70 mg/dl.
7 Consult with healthcare professional before turning off alarms. When alarms are turned off, notification will not be received during low or high glucose.
8 Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal-use, sensor-based glucose monitoring systems.
According to Abbott, this is the only iCGM system with optional real-time alarms that measures glucose levels every minute, meeting the highest level of accuracy standards5 over 14 days, including superior day one1 accuracy compared to the other iCGM and excellent accuracy and alarm performance at low end glucose levels.6 With a 14-day wear time, the FreeStyle Libre 2 system “is the longest-lasting, self-applied iCGM sensor currently available, eliminating the need for fingersticks2– and priced at a third of the cost of other CGM systems.”4
"We're thrilled to bring our next generation technology on our world-leading sensing platform to both children and adults with diabetes in the U.S.," said Jared Watkin, senior vice president, diabetes care, Abbott. "With unsurpassed 14-day accuracy and enhanced features including optional alarms at a fraction of the cost of other CGMs, Abbott's FreeStyle Libre 2 system will change the future of diabetes care in the U.S. the same way it has around the globe."
Using Bluetooth technology, the FreeStyle Libre 2 system automatically alerts users when their glucose is high or low without needing to scan the sensor. Users also have the option of turning off the customizable, real-time alarms.7 The system has a combined mean absolute relative difference (MARD), a measurement of performance for CGMs, of 9.3% (9.2% for adults and 9.7% for pediatrics).
"Innovations like FreeStyle Libre 2 will change the way people manage their diabetes, especially among children," said Larry Kurt Midyett, M.D., pediatric endocrinologist, Midwest Women's and Children's Specialty Group. "Using this technology can improve time in optimal glucose range and lower HbA1c because we can get a full picture of what a child's glucose levels are doing without having to disrupt their play or sleep with painful fingersticks. The alarms are a bonus because they provide parents a level of reassurance."
The FreeStyle Libre 2 next-generation sensor is worn on the back of the upper arm for up to 14 days and measures glucose every minute to help users and their healthcare providers make informed treatment decisions. With a one-second scan using a handheld reader, users can see their glucose reading, trend arrow and eight-hour history. It is also designed for use with a mobile app, which Abbott is working to bring to the U.S. market.
The FreeStyle Libre 2 system will be available in the coming weeks at participating pharmacies and durable medical equipment suppliers (DMEs) across the U.S. Abbott will offer the new system at the same price as the currently available FreeStyle Libre 14 day system, which was approved by the FDA in July 2018.
References:
1 Data on file, Abbott Diabetes Care.
2 Fingersticks are required if your glucose alarms and readings do not match symptoms or when you see Check Blood Glucose symbol during the first 12 hours.
3 Eeg-Olofsson, Katarina et al. Real-world study of FreeStyle Libre system among adults with Type 1 and Type 2 diabetes within the Swedish National Diabetes Register. Poster presented at ATTD, February 2020.
4 Based on a comparison of list prices of the FreeStyle Libre 14 day system versus competitors' CGM systems. FreeStyle Libre 2 system will be list priced the same rate as FreeStyle Libre 14 day system. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
5 Based on FDA iCGM special controls.
6 FreeStyle Libre 2 User Manual. Based on low glucose alarms set at 70 mg/dl.
7 Consult with healthcare professional before turning off alarms. When alarms are turned off, notification will not be received during low or high glucose.
8 Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal-use, sensor-based glucose monitoring systems.