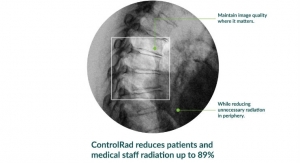
Globe Newswire11.08.19
Endonovo Therapeutics Inc. has appointed Dr. William Li as Strategic Advisor to the CEO. Dr. Li and Endonovo CEO Alan Collier will work collaboratively and strategically to explore growth strategies for Endonovo's contemplated market segments.
"Dr. Li is a globally recognized medical professional and a business visionary with a strong track record of success,” Collier stated. “Like us, he takes a long-term view of medical advances and believes industry can play a pivotal role in shaping the future. We look forward to working with Dr. Li to further extend our industry leadership by aligning Endonovo with each of the major market segments we can impact."
Dr. Li is CEO and co-founder of the Angiogenesis Foundation. He trained in the lab of Dr. Judah Folkman, pioneer of the angiogenesis field, and has been actively engaged in angiogenesis research and clinical development for 30 years. Under Dr. Li’s leadership, the foundation has developed a unique social enterprise model based on value-creating collaborations with leading biopharmaceutical and medical device companies.
Dr. Li is actively engaged in identifying unmet needs in the healthcare space for which clinical and cost effective technology can offer beneficial solutions. He is a graduate of Harvard, and completed his medical residency training at Massachusetts General Hospital in Boston. He serves as advisor and consultant to leading global public and private companies.
Dr. Li added, "I believe Endonovo has a strong technology platform with game changing potential as a player in the post-operative healing process, and is poised to go to the next level across a range of industry verticals. I look forward to working with Alan and the Endonovo team to evaluate the company’s operational and strategic opportunities.”
Endonovo Therapeutics Inc. is a commercial-stage developer of noninvasive wearable Electroceuticals therapeutic devices. The company's current portfolio of commercial and clinical-stage wearable Electroceuticals therapeutic devices addresses wound healing, pain, post-surgical pain and edema, cardiovascular disease, chronic kidney disease, and central nervous system (CNS) disorders, including traumatic brain injury (TBI), acute concussions, post-concussion syndrome and multiple sclerosis. The company's noninvasive Electroceutical therapeutic device, SofPulse, which uses pulsed short-wave radiofrequency at 27.12 MHz, has been cleared by the U.S. Food and Drug Administration and CE marked for the palliative treatment of soft tissue injuries and post-operative pain and edema and has CMS national coverage for the treatment of chronic wounds. The company's current portfolio of preclinical-stage Electroceuticals therapeutic devices addresses chronic kidney disease, liver disease non-alcoholic steatohepatitis (NASH), cardiovascular and peripheral artery disease (PAD), and ischemic stroke. The ompany's noninvasive, wearable Electroceuticals therapeutic devices work by restoring key electrochemical processes that initiate anti-inflammatory and growth factor cascades necessary for healing to occur.
"Dr. Li is a globally recognized medical professional and a business visionary with a strong track record of success,” Collier stated. “Like us, he takes a long-term view of medical advances and believes industry can play a pivotal role in shaping the future. We look forward to working with Dr. Li to further extend our industry leadership by aligning Endonovo with each of the major market segments we can impact."
Dr. Li is CEO and co-founder of the Angiogenesis Foundation. He trained in the lab of Dr. Judah Folkman, pioneer of the angiogenesis field, and has been actively engaged in angiogenesis research and clinical development for 30 years. Under Dr. Li’s leadership, the foundation has developed a unique social enterprise model based on value-creating collaborations with leading biopharmaceutical and medical device companies.
Dr. Li is actively engaged in identifying unmet needs in the healthcare space for which clinical and cost effective technology can offer beneficial solutions. He is a graduate of Harvard, and completed his medical residency training at Massachusetts General Hospital in Boston. He serves as advisor and consultant to leading global public and private companies.
Dr. Li added, "I believe Endonovo has a strong technology platform with game changing potential as a player in the post-operative healing process, and is poised to go to the next level across a range of industry verticals. I look forward to working with Alan and the Endonovo team to evaluate the company’s operational and strategic opportunities.”
Endonovo Therapeutics Inc. is a commercial-stage developer of noninvasive wearable Electroceuticals therapeutic devices. The company's current portfolio of commercial and clinical-stage wearable Electroceuticals therapeutic devices addresses wound healing, pain, post-surgical pain and edema, cardiovascular disease, chronic kidney disease, and central nervous system (CNS) disorders, including traumatic brain injury (TBI), acute concussions, post-concussion syndrome and multiple sclerosis. The company's noninvasive Electroceutical therapeutic device, SofPulse, which uses pulsed short-wave radiofrequency at 27.12 MHz, has been cleared by the U.S. Food and Drug Administration and CE marked for the palliative treatment of soft tissue injuries and post-operative pain and edema and has CMS national coverage for the treatment of chronic wounds. The company's current portfolio of preclinical-stage Electroceuticals therapeutic devices addresses chronic kidney disease, liver disease non-alcoholic steatohepatitis (NASH), cardiovascular and peripheral artery disease (PAD), and ischemic stroke. The ompany's noninvasive, wearable Electroceuticals therapeutic devices work by restoring key electrochemical processes that initiate anti-inflammatory and growth factor cascades necessary for healing to occur.