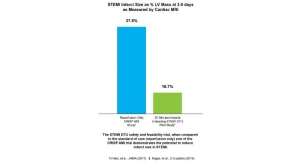
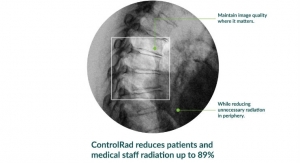
Business Wire10.08.19
Topcon has launched the Maestro2 Automated OCT/Fundus Camera, now with OCTA. Building on the success of its predecessor, the 3D OCT-1 Maestro, the new Maestro2 adds even more clinical utility to its multimodality platform.1
The Maestro2 is a fully automated OCT system that can capture high resolution non-mydriatic, true color fundus photography, OCT, and OCTA with the single press of a button. This multimodal system also now offers the Hood Report for the structure/function analysis of glaucoma. Additionally, it features a 360 degree rotating intuitive touchscreen, a small footprint and space-saving design.
First introduced to the industry in 2014, OCT Angiography, with its presentation of the vascular network of the retina, is rapidly growing in clinical acceptance. It has become a valuable aid in the detection and monitoring of key retinal pathologies such as Choroidal Neovascularization, Diabetic Retinopathy, microaneurysms, and more. Topcon's OCTA offering, for both its SS-OCT and SD-OCT models, demonstrates its investment in this technology across its platforms for utilization by multiple eye care specialists and generalists alike.
In addition to being extremely easy to use, the Maestro2 is equipped with clinical utilities. It features IMAGEnet 6 capture software for dynamic viewing of OCT and imaging data. Additionally, Topcon's exclusive PinPoint Registration precisely matches specified areas within OCT and OCTA scans upon the color fundus image. The Maestro2’s new follow-up scan feature scans the exact same location each patient visit, beneficial for follow-up visits and tracking diseases over time. A portfolio of reports for macula, anterior, and glaucoma allows the practitioner access to advanced diagnostic data.
John Trefethen, vice president of Global Marketing and Product Design for Topcon, stated, "The Maestro2 is the culmination of our efforts to deliver a powerful OCT system that not only features exceptional image quality and advanced diagnostic capabilities such as OCTA but also offers the practitioner workflow enhancements, detailed image analysis and reporting functions, and data management capabilities. With the addition of fully automated OCTA, the Maestro2 truly is the most comprehensive OCT system in the marketplace, and we are excited to bring this innovative technology to eye care specialists around the globe.”
Topcon is a diagnostic device manufacturer within the worldwide eye care community. It introduced the world’s first commercial back-of-the-eye Spectral Domain (SD) and multimodal Swept Source (SS) optical coherence tomography (OCT) systems, which have driven innovation in eye care. More recently, to develop the most efficient, pragmatic solutions, Topcon formed a new strategic division, Topcon Healthcare Solutions, whose primary objective is to create software solutions for the eye care industry and beyond. The company's products enable the collection and visualization of a wide range of imaging and clinical data while providing quantitative and clinical analysis capabilities.
Topcon’s software gives clinicians access to patient exam data captured from OCTs, Visual Fields, Fundus Cameras, and other Topcon and third-party devices. Topcon leverages its new data management system called Topcon Harmony, where practitioners gain access to both DICOM and non-DICOM information stored in a central, cloud-based environment. Additionally, Topcon now provides an integrated service that connects practitioners to a network of reading services to assist in the management of sight-threatening eye diseases.
Reference
1 Maestro2 and OCT Angiography are not available for sale in all countries. Please check with your local distributor for availability in your country.
The Maestro2 is a fully automated OCT system that can capture high resolution non-mydriatic, true color fundus photography, OCT, and OCTA with the single press of a button. This multimodal system also now offers the Hood Report for the structure/function analysis of glaucoma. Additionally, it features a 360 degree rotating intuitive touchscreen, a small footprint and space-saving design.
First introduced to the industry in 2014, OCT Angiography, with its presentation of the vascular network of the retina, is rapidly growing in clinical acceptance. It has become a valuable aid in the detection and monitoring of key retinal pathologies such as Choroidal Neovascularization, Diabetic Retinopathy, microaneurysms, and more. Topcon's OCTA offering, for both its SS-OCT and SD-OCT models, demonstrates its investment in this technology across its platforms for utilization by multiple eye care specialists and generalists alike.
In addition to being extremely easy to use, the Maestro2 is equipped with clinical utilities. It features IMAGEnet 6 capture software for dynamic viewing of OCT and imaging data. Additionally, Topcon's exclusive PinPoint Registration precisely matches specified areas within OCT and OCTA scans upon the color fundus image. The Maestro2’s new follow-up scan feature scans the exact same location each patient visit, beneficial for follow-up visits and tracking diseases over time. A portfolio of reports for macula, anterior, and glaucoma allows the practitioner access to advanced diagnostic data.
John Trefethen, vice president of Global Marketing and Product Design for Topcon, stated, "The Maestro2 is the culmination of our efforts to deliver a powerful OCT system that not only features exceptional image quality and advanced diagnostic capabilities such as OCTA but also offers the practitioner workflow enhancements, detailed image analysis and reporting functions, and data management capabilities. With the addition of fully automated OCTA, the Maestro2 truly is the most comprehensive OCT system in the marketplace, and we are excited to bring this innovative technology to eye care specialists around the globe.”
Topcon is a diagnostic device manufacturer within the worldwide eye care community. It introduced the world’s first commercial back-of-the-eye Spectral Domain (SD) and multimodal Swept Source (SS) optical coherence tomography (OCT) systems, which have driven innovation in eye care. More recently, to develop the most efficient, pragmatic solutions, Topcon formed a new strategic division, Topcon Healthcare Solutions, whose primary objective is to create software solutions for the eye care industry and beyond. The company's products enable the collection and visualization of a wide range of imaging and clinical data while providing quantitative and clinical analysis capabilities.
Topcon’s software gives clinicians access to patient exam data captured from OCTs, Visual Fields, Fundus Cameras, and other Topcon and third-party devices. Topcon leverages its new data management system called Topcon Harmony, where practitioners gain access to both DICOM and non-DICOM information stored in a central, cloud-based environment. Additionally, Topcon now provides an integrated service that connects practitioners to a network of reading services to assist in the management of sight-threatening eye diseases.
Reference
1 Maestro2 and OCT Angiography are not available for sale in all countries. Please check with your local distributor for availability in your country.