Beckman Coulter06.14.19
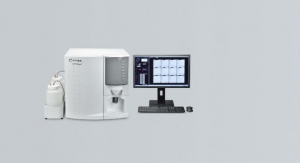
Beckman Coulter, a global provider of clinical diagnostics, has announced the latest addition to its automation portfolio: The DxA 5000 total laboratory automation solution has achieved European CE Mark and China Food and Drug Administration approval. In today’s healthcare environment, laboratories are highly focused on enhancing patient care by driving faster turnaround time, delivering quality results and improving laboratory operations. The DxA 5000 helps laboratories meet these challenges through a collection of patented innovations that deliver rapid and consistent turnaround time, provide a new level of pre-analytical sample quality detection, and reduce the number of manual processing steps to significantly improve laboratory efficiency.
Reporting results faster to physicians can positively impact patient outcomes. In the laboratory, specimen centrifugation is typically the most time-consuming pre-analytical activity.1,2 The DxA 5000 sets a new standard by utilizing a universal centrifugation protocol that significantly reduces the pre-analytical processing time by up to 73 percent for connected analyzers across multiple disciplines.3
Additionally, the DxA 5000 supports laboratories in delivering highly consistent turnaround time to their physicians. Leveraging first-of-its-kind dynamic system software, the DxA 5000 utilizes Intelligent Routing to bring automated patient-centric workflow to the laboratory. By understanding the tests requested, sample volume available and real-time analyzer capacity and status, the DxA 5000 continuously calculates the most expeditious route for every patient sample—both STAT and routine.
“The DxA 5000 is a breakthrough solution in total laboratory automation, focused on enhancing the efforts of our laboratory partners to positively impact patient outcomes,” said John Blackwood, Beckman Coulter’s senior vice president of products and services. “By accelerating and reducing variability in turnaround time, laboratories can more efficiently provide physicians with the critical information they need to best manage patient care in an environment where every minute matters.”
Unmatched Quality Assessment and Improved Efficiency
Research shows errors that occur in the pre-analytical phase of testing may contribute up to 75 percent of erroneous test results, with 26 percent possibly having adverse effects on patient care.4 Moreover, a vast majority of the factors causing erroneous results occur outside the laboratory, including inadequate volume, mislabeled samples and incorrect tube type.5,6,7,8 The DxA 5000 is designed with a sharp focus on sample quality assessment, screening each sample at multiple points to help laboratories substantially reduce the risk of errors. In three seconds, the system detects patient tube parameters such as volume, sample identification, tube type, cap color, orders pending and tube weight. The system is also designed to check for sample volume at three separate points: pre-centrifugation, post-centrifugation and prior to sample storage to continually ensure sufficient volume is available for the tests ordered. Together, these quality assessments reduce the potential for release of erroneous results and can help accelerate time to result by quickly alerting the laboratory when a new patient sample is needed.
In addition to improving sample quality, laboratories also require workflow solutions that help them manage high volumes of outpatient, outreach and network samples, while processing urgent requests for both patient wards and emergency departments. The DxA 5000 simultaneously manages high volumes of multiple discipline requests, with a large variety of patient sample tubes and sizes, without impact to overall system throughput or STAT turnaround time. By automating sample processing steps, the DxA 5000 supports laboratories in delivering a higher number of results per hour with the same staffing level.
“Based on research and work performed with our laboratory partners, sample processing steps are shown to make up approximately 70 percent of a laboratory’s labor hours,” continued Blackwood. “The DxA 5000 significantly reduces the number of manual steps in sample processing to as few as one. From sample accessioning and quality assessment to add-on test management and sample disposal, the DxA 5000 enables laboratory professionals to deliver high-quality results and improve efficiency, empowering them to focus their efforts and skills on managing patient sample exceptions.”
The first in a suite of DxA systems currently in development, the DxA 5000 enhances Beckman Coulter’s comprehensive portfolio of scalable solutions and is a key component of its vision to bring workflow automation to laboratories of all sizes. This launch is the culmination of years of customer-inspired design and rigorous reliability testing and strengthens Beckman Coulter’s ongoing commitment to providing solutions that deliver accurate results with fast, consistent turnaround time, better detection of pre-analytical errors, and more efficient workflows designed to fit the needs of any-sized laboratory.
A 510(k) submission for the DxA 5000 is pending clearance with the U.S. Food and Drug Administration and is not yet available in the United States.
Beckman Coulter is committed to advancing healthcare for every person by applying the power of science, technology, and the passion and creativity of its teams to enhance the diagnostic laboratory’s role in improving healthcare outcomes. Its diagnostic systems are used in complex biomedical testing, and are found in hospitals, reference laboratories and physician office settings around the globe. Beckman Coulter offers a combination of people, processes and solutions designed to elevate the performance of clinical laboratories and healthcare networks. An operating company of Danaher Corporation since 2011, Beckman Coulter is headquartered in Brea, Calif., and has more than 11,000 global associates.
References
1 Estey CA, Felder RA. Clinical evaluation of serial blood processing at point-of-care. Clin Chem 1997;43:360-2
2 Roberts T, Smith M, Roberts B. Observations in centrifugation: application to centrifuge development. Clin Chem 1999;45:1889-97
3 Beckman Coulter Study, “Reducing the turnaround time of the pre-analytical phase by application of a rapid centrifugation profile.” S. Frankenberger, et al.
4 Green. Sol. F., Clinical Biochemistry 46 (2013), 1175–1179.
5 J Clin Diagn Res, Nov; 7(11): 2491–2493. 2013.
6 LabMedicine, 41, 89-92. (2010).
7 Clinical Chemistry 53:7, 1338–1342 (2007).
8 DOI 10.1515/cclm-2013-0597. Clin Chem Lab Med 2013; aop.
Reporting results faster to physicians can positively impact patient outcomes. In the laboratory, specimen centrifugation is typically the most time-consuming pre-analytical activity.1,2 The DxA 5000 sets a new standard by utilizing a universal centrifugation protocol that significantly reduces the pre-analytical processing time by up to 73 percent for connected analyzers across multiple disciplines.3
Additionally, the DxA 5000 supports laboratories in delivering highly consistent turnaround time to their physicians. Leveraging first-of-its-kind dynamic system software, the DxA 5000 utilizes Intelligent Routing to bring automated patient-centric workflow to the laboratory. By understanding the tests requested, sample volume available and real-time analyzer capacity and status, the DxA 5000 continuously calculates the most expeditious route for every patient sample—both STAT and routine.
“The DxA 5000 is a breakthrough solution in total laboratory automation, focused on enhancing the efforts of our laboratory partners to positively impact patient outcomes,” said John Blackwood, Beckman Coulter’s senior vice president of products and services. “By accelerating and reducing variability in turnaround time, laboratories can more efficiently provide physicians with the critical information they need to best manage patient care in an environment where every minute matters.”
Unmatched Quality Assessment and Improved Efficiency
Research shows errors that occur in the pre-analytical phase of testing may contribute up to 75 percent of erroneous test results, with 26 percent possibly having adverse effects on patient care.4 Moreover, a vast majority of the factors causing erroneous results occur outside the laboratory, including inadequate volume, mislabeled samples and incorrect tube type.5,6,7,8 The DxA 5000 is designed with a sharp focus on sample quality assessment, screening each sample at multiple points to help laboratories substantially reduce the risk of errors. In three seconds, the system detects patient tube parameters such as volume, sample identification, tube type, cap color, orders pending and tube weight. The system is also designed to check for sample volume at three separate points: pre-centrifugation, post-centrifugation and prior to sample storage to continually ensure sufficient volume is available for the tests ordered. Together, these quality assessments reduce the potential for release of erroneous results and can help accelerate time to result by quickly alerting the laboratory when a new patient sample is needed.
In addition to improving sample quality, laboratories also require workflow solutions that help them manage high volumes of outpatient, outreach and network samples, while processing urgent requests for both patient wards and emergency departments. The DxA 5000 simultaneously manages high volumes of multiple discipline requests, with a large variety of patient sample tubes and sizes, without impact to overall system throughput or STAT turnaround time. By automating sample processing steps, the DxA 5000 supports laboratories in delivering a higher number of results per hour with the same staffing level.
“Based on research and work performed with our laboratory partners, sample processing steps are shown to make up approximately 70 percent of a laboratory’s labor hours,” continued Blackwood. “The DxA 5000 significantly reduces the number of manual steps in sample processing to as few as one. From sample accessioning and quality assessment to add-on test management and sample disposal, the DxA 5000 enables laboratory professionals to deliver high-quality results and improve efficiency, empowering them to focus their efforts and skills on managing patient sample exceptions.”
The first in a suite of DxA systems currently in development, the DxA 5000 enhances Beckman Coulter’s comprehensive portfolio of scalable solutions and is a key component of its vision to bring workflow automation to laboratories of all sizes. This launch is the culmination of years of customer-inspired design and rigorous reliability testing and strengthens Beckman Coulter’s ongoing commitment to providing solutions that deliver accurate results with fast, consistent turnaround time, better detection of pre-analytical errors, and more efficient workflows designed to fit the needs of any-sized laboratory.
A 510(k) submission for the DxA 5000 is pending clearance with the U.S. Food and Drug Administration and is not yet available in the United States.
Beckman Coulter is committed to advancing healthcare for every person by applying the power of science, technology, and the passion and creativity of its teams to enhance the diagnostic laboratory’s role in improving healthcare outcomes. Its diagnostic systems are used in complex biomedical testing, and are found in hospitals, reference laboratories and physician office settings around the globe. Beckman Coulter offers a combination of people, processes and solutions designed to elevate the performance of clinical laboratories and healthcare networks. An operating company of Danaher Corporation since 2011, Beckman Coulter is headquartered in Brea, Calif., and has more than 11,000 global associates.
References
1 Estey CA, Felder RA. Clinical evaluation of serial blood processing at point-of-care. Clin Chem 1997;43:360-2
2 Roberts T, Smith M, Roberts B. Observations in centrifugation: application to centrifuge development. Clin Chem 1999;45:1889-97
3 Beckman Coulter Study, “Reducing the turnaround time of the pre-analytical phase by application of a rapid centrifugation profile.” S. Frankenberger, et al.
4 Green. Sol. F., Clinical Biochemistry 46 (2013), 1175–1179.
5 J Clin Diagn Res, Nov; 7(11): 2491–2493. 2013.
6 LabMedicine, 41, 89-92. (2010).
7 Clinical Chemistry 53:7, 1338–1342 (2007).
8 DOI 10.1515/cclm-2013-0597. Clin Chem Lab Med 2013; aop.